Plasma Physics Definitions
... Before application of the potential, gas molecules are electrically neutral and the gas at room temperature will contain very few if any charged particles. Occasionally however, a free electron may be released from a molecule by the interaction of, for example, a cosmic ray or other natural radiatio ...
... Before application of the potential, gas molecules are electrically neutral and the gas at room temperature will contain very few if any charged particles. Occasionally however, a free electron may be released from a molecule by the interaction of, for example, a cosmic ray or other natural radiatio ...
Last 4 Digits of USC ID:____ ____ ____ ____ Dr.
... 7. (10 pt) What pH must be maintained by a buffer solution so that no more than 0.010% of the Mg2+ present in 0.360 M MgCl2 (aq) remains in solution following precipitation of Mg(OH)2 (s)? Ksp for Mg(OH)2 = 8.9 x 10-12. ...
... 7. (10 pt) What pH must be maintained by a buffer solution so that no more than 0.010% of the Mg2+ present in 0.360 M MgCl2 (aq) remains in solution following precipitation of Mg(OH)2 (s)? Ksp for Mg(OH)2 = 8.9 x 10-12. ...
Unit 4 - Dorman High School
... What is the difference in electronegativity that needs to occur in order for an ionic bond to occur? III. Bond Polarity If the difference in electronegativity is large enough the bond between the atoms will have polarity. What is this called? Any diatomic molecule will have a dipole moment. When wil ...
... What is the difference in electronegativity that needs to occur in order for an ionic bond to occur? III. Bond Polarity If the difference in electronegativity is large enough the bond between the atoms will have polarity. What is this called? Any diatomic molecule will have a dipole moment. When wil ...
The flame ionization detector: A theoretical approach
... initially, and eventually may be collected (possibly after chemical re-arrangement or exchange in the flame). These concepts, if true, lead to several interesting consequences. Let us suppose, for convenience, that the jet is positive and that the field applied is sufficient to cause complete separa ...
... initially, and eventually may be collected (possibly after chemical re-arrangement or exchange in the flame). These concepts, if true, lead to several interesting consequences. Let us suppose, for convenience, that the jet is positive and that the field applied is sufficient to cause complete separa ...
Challenge - ChemistryIBWYA
... they react with the solvent to form ions. Instead of a simple dissociation, the chemical reaction produces the charged particles. A solution of hydrochloric acid in water is one such example: Substances that tend to ionize completely include strong acids, strong bases, and ionic compounds (salts). S ...
... they react with the solvent to form ions. Instead of a simple dissociation, the chemical reaction produces the charged particles. A solution of hydrochloric acid in water is one such example: Substances that tend to ionize completely include strong acids, strong bases, and ionic compounds (salts). S ...
Jean-Charles Matéo-Vélez - Institut de Mathématiques de Toulouse
... acceleration of positive and negative ions due to the strong electric field (Lorentz force), collisions with the neutral molecules of air, ionic wind effect, competition between positive and negative ionic wind. ...
... acceleration of positive and negative ions due to the strong electric field (Lorentz force), collisions with the neutral molecules of air, ionic wind effect, competition between positive and negative ionic wind. ...
Nature of Acids and Bases
... ionization of water, which of the following is true? (1) The forward reaction forming ions from water is favored. (2) The concentration of ions in pure water is high. (3) The concentration of hydronium in pure water is higher than the concentration of hydroxide. (4) The concentration of ions in pure ...
... ionization of water, which of the following is true? (1) The forward reaction forming ions from water is favored. (2) The concentration of ions in pure water is high. (3) The concentration of hydronium in pure water is higher than the concentration of hydroxide. (4) The concentration of ions in pure ...
Webquest Review - Harrison High School
... C: View “VSEPR – Valence Shell Electron Pair Repulsion.” 14. How many areas of high electron density surround the central atom in NH3? There are 4 electron density sites on the nitrogen. 15. What is the molecular shape of SO3 molecule? The shape of a sulfur trioxide molecule would be trigonal planar ...
... C: View “VSEPR – Valence Shell Electron Pair Repulsion.” 14. How many areas of high electron density surround the central atom in NH3? There are 4 electron density sites on the nitrogen. 15. What is the molecular shape of SO3 molecule? The shape of a sulfur trioxide molecule would be trigonal planar ...
Chapter 7 - HCC Learning Web
... • Have highly negative electron affinities – Exist as anions in nature ...
... • Have highly negative electron affinities – Exist as anions in nature ...
NOMENCLATURE OF IONIC COMPOUNDS CHEMISTRY 1405
... One may be tempted to put the oxidatiion number of Barium within parenthesis such as Barium (II) and this is incorrect. Oxidation number is expressed in parenthesis only for transistion metal ions or metal ions which show variable oxidation numbers. Barium belongs to group 2 and all elements in grou ...
... One may be tempted to put the oxidatiion number of Barium within parenthesis such as Barium (II) and this is incorrect. Oxidation number is expressed in parenthesis only for transistion metal ions or metal ions which show variable oxidation numbers. Barium belongs to group 2 and all elements in grou ...
File
... E) A scientific law summarizes a series of related observations 3) Which of the following statements about the phases of matter is TRUE? A) In both solids and liquids, the atoms or molecules pack closely to one another. B) Solids are highly compressible. C) Gaseous substances have long-range repeati ...
... E) A scientific law summarizes a series of related observations 3) Which of the following statements about the phases of matter is TRUE? A) In both solids and liquids, the atoms or molecules pack closely to one another. B) Solids are highly compressible. C) Gaseous substances have long-range repeati ...
1.1 Safety in the Science Classroom
... The positive ions attract all of the negative ions, and vice versa. In the example of table salt (NaCl) the one-to-one ratio of ions results in a simple square-shaped ionic cyrstal: ...
... The positive ions attract all of the negative ions, and vice versa. In the example of table salt (NaCl) the one-to-one ratio of ions results in a simple square-shaped ionic cyrstal: ...
Hinge Point Questions
... Which statement best explains why chlorine has a mass of 35.5? a) 35.5 is the average mass of all the isotopes of chlorine. b) Chlorine and similar elements have ½ a neutron. c) Some chlorine atoms have a mass of 35, others have different masses. d) Different chlorine atoms have different numbers o ...
... Which statement best explains why chlorine has a mass of 35.5? a) 35.5 is the average mass of all the isotopes of chlorine. b) Chlorine and similar elements have ½ a neutron. c) Some chlorine atoms have a mass of 35, others have different masses. d) Different chlorine atoms have different numbers o ...
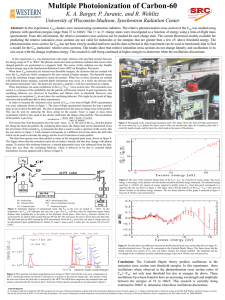
Figure 4 - University of Wisconsin–Madison
... Abstract: In this experiment, C60 clusters were ionized using synchrotron radiation. The relative photoionization cross section of the C60 was studied using photons with specified energies range from 37 to 160eV. The 1+ to 3+ charge states were investigated as a function of energy using a time-of-fl ...
... Abstract: In this experiment, C60 clusters were ionized using synchrotron radiation. The relative photoionization cross section of the C60 was studied using photons with specified energies range from 37 to 160eV. The 1+ to 3+ charge states were investigated as a function of energy using a time-of-fl ...
Document
... will have a higher boiling point than that of a pure solvent after the addition of a dissolved solute. The change in boiling point can be determined by the equation ΔTB.P.= i ·Kb ·m, where m is the molality of the solute(mol/kg), i is the Van 't Hoff factor (the number of dissolved particles the sol ...
... will have a higher boiling point than that of a pure solvent after the addition of a dissolved solute. The change in boiling point can be determined by the equation ΔTB.P.= i ·Kb ·m, where m is the molality of the solute(mol/kg), i is the Van 't Hoff factor (the number of dissolved particles the sol ...
Class 9 CBSE Test paper Solved Chapter 3: Atoms and...
... the mass of oxygen gas that would be required to react completely with 6 gm carbon. Solution: As per the law of definite proportional : The composition of a compound always remains fixed and it is independent to the source from which the compound is obtained. Let the mass of oxygen gas that would be ...
... the mass of oxygen gas that would be required to react completely with 6 gm carbon. Solution: As per the law of definite proportional : The composition of a compound always remains fixed and it is independent to the source from which the compound is obtained. Let the mass of oxygen gas that would be ...
Lecture 02
... • Molar concentrations: C+ and C• In the absent of electric field; – Disregard the random motion of ions and imagine ions as stationary point charges embedded in the solvent. ...
... • Molar concentrations: C+ and C• In the absent of electric field; – Disregard the random motion of ions and imagine ions as stationary point charges embedded in the solvent. ...
Chapter one
... a. When the rate of forward reaction is = to the rate of the reverse reaction - chemical equilibrium is established. ...
... a. When the rate of forward reaction is = to the rate of the reverse reaction - chemical equilibrium is established. ...
pH scale learning goals
... Learning goals for pH scale Students will be able to use pH Scale to • Write descriptions that demonstrate the use of pH and/or relative hydronium and hydroxide ions as shown in the simulation to: A. Determine if a liquid is acidic or basic B. Place liquids in relative order of acidity or basicity ...
... Learning goals for pH scale Students will be able to use pH Scale to • Write descriptions that demonstrate the use of pH and/or relative hydronium and hydroxide ions as shown in the simulation to: A. Determine if a liquid is acidic or basic B. Place liquids in relative order of acidity or basicity ...
Lecture 19
... concentration are important factors in method development. Interactions between these factors can also be complex. For example, the effect of temperature becomes a very critical factor and columns are almost always temperature controlled with ion-pair chromatography. ...
... concentration are important factors in method development. Interactions between these factors can also be complex. For example, the effect of temperature becomes a very critical factor and columns are almost always temperature controlled with ion-pair chromatography. ...
Polarizability
... Polarizability usually increases as the number of electrons in an atom or molecule increases. Therefore, the strength of dispersion forces tends to increase with increasing atomic or molecular size. ...
... Polarizability usually increases as the number of electrons in an atom or molecule increases. Therefore, the strength of dispersion forces tends to increase with increasing atomic or molecular size. ...
Chem 11 Study Guide SCH3U Unit 1 Definitions: SATP: Standard
... • Using electronegativity number and VSEPR theory, you can figure out if a molecule is polar covalent, non-polar covalent, or ionic. • If the difference in elec-ity is <0.4 (less than), it is non-polar covalent (if you already checked it’s covalent in the first place lol) • Btwn 0.5 and 1.7 AND a VS ...
... • Using electronegativity number and VSEPR theory, you can figure out if a molecule is polar covalent, non-polar covalent, or ionic. • If the difference in elec-ity is <0.4 (less than), it is non-polar covalent (if you already checked it’s covalent in the first place lol) • Btwn 0.5 and 1.7 AND a VS ...
Ion source

An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.