* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Challenge - ChemistryIBWYA
Rutherford backscattering spectrometry wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Ionic liquid wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Electrochemistry wikipedia , lookup
Electrolysis of water wikipedia , lookup
Acid–base reaction wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Ionic compound wikipedia , lookup
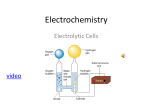
ICT Design DC DPP CE MS PS(a) GRADE 11 & 12- CHMISTRY INVESTIGATION Strength of Electrolytic solutions Challenge Test various concentrations of electrolytic solutions in order to determine the relationships among degree of dissociation, solute concentration, and electrolytic strength. 1.0Equipment and Materials • Copper wire with crocodile clips • Cardboard or foam board • Two carbon electrodes • Plastic containers 2 • 100 ml of each of the following type of test solutions • wash bottle and waste container • Multimeter ( 0.3mA- 300mA) find the uncertainty of the instrument ? Following solutions (in 150-mL beakers): 1) 0.5 M hydrochloric acid (HCl) 2) 0.5 M acetic acid (CH3CO2H) 3) 0.5 M ammonium hydroxide (NH4OH) 4) 0.5 M calcium hydroxide (Ca(OH)2) 5) Tap water 6) 0.5 M ammonium chloride (NH4Cl) 7) water, distilled 2.1 Safety Precautions Handle solutions carefully! Concentrated acids and bases can burn skin. Wear gloves and lab aprons and clean up spills with care. Avoid breathing fumes from any of the test solutions. Wear safety glasses and follow standard laboratory safety procedures. 1 2.2 Background Electrolyte solutions conduct electrical current due to the presence of charged particles, or ions, in the solution. [Charged particles are created when a substance either dissociates in or reacts with the solvent (usually water) to form ions.] The more ions the solution contains, the greater the amount of electrical current the solution can carry—in other words, the better a conductor it is. Substances that dissolve to form good conductive solutions are called strong electrolytes. They are usually ionic compounds, breaking apart when dissolved to exist in their ionized form. As a result, the solution contains no intact ionic compounds, only free ions. Since measuring a solution’s conductivity gives an indication of the number of ions in the solution, a high conductivity reading indicates a strong electrolyte. Some molecular compounds, notably acids and bases, can also form solutions with high conductivity, because when dissolved they react with the solvent to form ions. Instead of a simple dissociation, the chemical reaction produces the charged particles. A solution of hydrochloric acid in water is one such example: Substances that tend to ionize completely include strong acids, strong bases, and ionic compounds (salts). Substances that are weak electrolytes are molecular compounds that dissociate only partially in solution. When dissolved, a limited number of ions do form, but intact molecules also exist to some degree in the solution. The solutions formed by weak electrolytes are poor conductors of electricity. Because fewer ions are present, measurements of their conductivity will be lower than for solutions of strong electrolytes. A substance can only form ions in solution if it first dissolves, so any electrolyte (strong or weak) must be at least partially soluble—that is, able to be dissolved by the solvent. However, there are substances that dissolve without forming anyions. These substances are soluble but are nonelectrolytes. What do you think the conductivity of such a solution would be? What if a substance is only partially soluble—in other words, it does not dissolve well? It may still be a strong electrolyte, if the amount that can dissolve dissociates completely. Although only a small amount of the substance dissolves, if that amount exists solely as ions, the substance is still classified as a strong electrolyte. Some salts, such as barium hydroxide, fall into this category. Because Ba(OH)2 is only slightly soluble in water, a concentrated aqueous solution cannot be made, but a dilute aqueous barium hydroxide solution will still conduct electricity due to the presence of Ba+ and OH- ions. 2 2.3 Equipment set up 2.3.1 Connect the electrode as per the fig1. 2.3.2 Negative lead connect to negative terminal of the dry cell battery .connect switch closer to negative electrode. 2.3.3 Connect multimeter terminal to positive terminal of the battery 2.3.4 Finally connect the remaining terminal of the meter to positive electrode .as per fig 2 Multimeter Battery Make contact switch Carbon Electrode Electrolytic solution Circuit diagram Fig 2 3 2.3.5 2.3.6 2.3.7 2.3.8 2.3.9 Pour 50ml of the solution in to the plastic container. Male the switch on and complete the electrical circuit . Record the ammeter reading . Clean the plastic container with distilled water . Step 2.3.5 to 2.3.8 with 6 different solutions . 4