Unit 4 Compounds, Naming, Formula Writing
... masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers. ...
... masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers. ...
Chapter 9: Chemical Quantities
... - given moles of a reactant or product you need to be able to use the stoichiometric relationships given in the balanced chemical equation to convert to moles of any other reactant or product -pictorial representations of chemical reactions ...
... - given moles of a reactant or product you need to be able to use the stoichiometric relationships given in the balanced chemical equation to convert to moles of any other reactant or product -pictorial representations of chemical reactions ...
CHAPTER 7 READING GUIDE – IONIC COMPOUNDS AND METALS
... 28. __________________, which are small numbers to the lower right of a symbol, represent the number of ______________ of each element in an ionic compound. 29. Many ionic compounds contain ___________________ ions, which are ions made up of more than one atom. 30. Because a polyatomic ion exists a ...
... 28. __________________, which are small numbers to the lower right of a symbol, represent the number of ______________ of each element in an ionic compound. 29. Many ionic compounds contain ___________________ ions, which are ions made up of more than one atom. 30. Because a polyatomic ion exists a ...
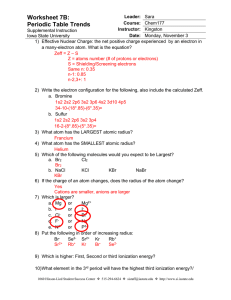
worksheet 7b answers - Iowa State University
... Iowa State University 1) Effective Nuclear Charge: the net positive charge experienced by an electron in a many-electron atom. What is the equation? Zeff = Z – S Z = atoms number (# of protons or electrons) S = Shielding/Screening electrons Same n: 0.35 n-1: 0.85 n-2,3+: 1 ...
... Iowa State University 1) Effective Nuclear Charge: the net positive charge experienced by an electron in a many-electron atom. What is the equation? Zeff = Z – S Z = atoms number (# of protons or electrons) S = Shielding/Screening electrons Same n: 0.35 n-1: 0.85 n-2,3+: 1 ...
Chemical Properties of Water - Part 2
... Many substances, such as household sugar, dissolve in water. That is, their molecules separate from each other, each becoming surrounded by water molecules. ...
... Many substances, such as household sugar, dissolve in water. That is, their molecules separate from each other, each becoming surrounded by water molecules. ...
Weak Acids and Bases Practice -- Chemistry 121A
... Weak Acids and Bases Practice -- Chemistry 121A Hanson OK, what’s new here really is just percent ionization. The way to think of this is that it is just a form of percent yield. It runs from 0% (all reactants) to 100% (all products). This is simply more convenient than Q, which runs from 0 to infin ...
... Weak Acids and Bases Practice -- Chemistry 121A Hanson OK, what’s new here really is just percent ionization. The way to think of this is that it is just a form of percent yield. It runs from 0% (all reactants) to 100% (all products). This is simply more convenient than Q, which runs from 0 to infin ...
FE Review Chemistry - UTSA College of Engineering
... Periodic Trends Electronegativity Ionization Energy ...
... Periodic Trends Electronegativity Ionization Energy ...
namimg compounds
... In the early days of chemistry, there was no system for the naming of compounds. Chemists used common names like bicarb of soda, quicklime, milk of magnesia, Epsom salts, spirits of salt, and laughing gas to describe compounds. As the number of named compounds increased it was obvious that if such c ...
... In the early days of chemistry, there was no system for the naming of compounds. Chemists used common names like bicarb of soda, quicklime, milk of magnesia, Epsom salts, spirits of salt, and laughing gas to describe compounds. As the number of named compounds increased it was obvious that if such c ...
Chemistry 150 - CSUB Home Page
... 2. Which of the following list the elements K, Li, Be, C, and F in order of increasing 1st Ionization Energy? (1 point, circle only one answer) a. b. ...
... 2. Which of the following list the elements K, Li, Be, C, and F in order of increasing 1st Ionization Energy? (1 point, circle only one answer) a. b. ...
Ion Exchange
... (ionic) interactions. The stationary phase surface displays ionic functional groups (R-X) that interact with analyte ions of opposite charge. An ion-exchange resin or ion-exchange polymer is an insoluble matrix normally in the form of small (1–2 mm diameter) beads fabricated from an organic polymer ...
... (ionic) interactions. The stationary phase surface displays ionic functional groups (R-X) that interact with analyte ions of opposite charge. An ion-exchange resin or ion-exchange polymer is an insoluble matrix normally in the form of small (1–2 mm diameter) beads fabricated from an organic polymer ...
ionization 12.3.1
... and electronic states and the electron has zero potential and kinetic energy. Electron energy The potential difference through which electrons are accelerated before they are used to bring about electron ionization. Fast atom bombardment ionization This term refers to the ionization of any species b ...
... and electronic states and the electron has zero potential and kinetic energy. Electron energy The potential difference through which electrons are accelerated before they are used to bring about electron ionization. Fast atom bombardment ionization This term refers to the ionization of any species b ...
EXAM # 1
... 4) How can mass spectrometry and atomic emission spectroscopy be used to determine the molecular formula of an unknown? AES can monitor the presence of up to 55 elements in an unknown sample by the observation of the presence/absence of the unique pattern of atomic emission bands for each element. S ...
... 4) How can mass spectrometry and atomic emission spectroscopy be used to determine the molecular formula of an unknown? AES can monitor the presence of up to 55 elements in an unknown sample by the observation of the presence/absence of the unique pattern of atomic emission bands for each element. S ...
Chpater 5.3 PPT
... to the edge of the outer orbital. Edge of outer orbital not well defined Use identical bonded atoms – ½ of the distance between the nuclei ...
... to the edge of the outer orbital. Edge of outer orbital not well defined Use identical bonded atoms – ½ of the distance between the nuclei ...
Mass Spectroscopy
... Separation of Ions • Only the cations are deflected by the magnetic field. • Amount of deflection depends on m/z. • The detector signal is proportional to the number of ions hitting it. • By varying the magnetic field, ions of all masses are collected and counted. => ...
... Separation of Ions • Only the cations are deflected by the magnetic field. • Amount of deflection depends on m/z. • The detector signal is proportional to the number of ions hitting it. • By varying the magnetic field, ions of all masses are collected and counted. => ...
Acid-Base Theories Arrhenius Acids and Bases • An acid is a
... between an electron-pair donor and an electron-pair acceptor. ...
... between an electron-pair donor and an electron-pair acceptor. ...
Periodic Trends
... occurs is related to the number of valence electrons. First ionization energy increases from left to right across a period. First ionization energy decreases down a group because atomic size increases and less energy is required to remove an electron farther from the nucleus. ...
... occurs is related to the number of valence electrons. First ionization energy increases from left to right across a period. First ionization energy decreases down a group because atomic size increases and less energy is required to remove an electron farther from the nucleus. ...
Class 1
... Chemical ionization (CI) Electrospray ionization (ESI) Atmospheric pressure chemical ionization (APCI) Matrix-assisted laser-desorption ionization (MALDI) ...
... Chemical ionization (CI) Electrospray ionization (ESI) Atmospheric pressure chemical ionization (APCI) Matrix-assisted laser-desorption ionization (MALDI) ...
ionization chamber
... If the applied voltage is further increased, gas amplification is so great that a single ionizing particle produces a ionization Avalanche. Each output pulse of current has the same magnitude and no longer reflects any properties of the incident radiation. ...
... If the applied voltage is further increased, gas amplification is so great that a single ionizing particle produces a ionization Avalanche. Each output pulse of current has the same magnitude and no longer reflects any properties of the incident radiation. ...
Combining and Choosing Analytical Techniques
... substance, this can be used to identify the substance from an on-line database or to give information about the structure of a new or unknown compound. ...
... substance, this can be used to identify the substance from an on-line database or to give information about the structure of a new or unknown compound. ...
IONIZATION METHODS IN MASS SPECTROMETRY
... rather than to a beam of energetic atoms or ions. c) The laser light is applied in pulses of short duration in contrast to exposure to a continuous beam of energetic atoms or ions. d) The analyte is ionized by energy transfer from the matrix rather than being "sputtered or ripped" from a liquid matr ...
... rather than to a beam of energetic atoms or ions. c) The laser light is applied in pulses of short duration in contrast to exposure to a continuous beam of energetic atoms or ions. d) The analyte is ionized by energy transfer from the matrix rather than being "sputtered or ripped" from a liquid matr ...
Ionization methods - 2-CI - Florida International University
... Excess of energy after ionization is 15.755 eV Excess of energy will transfer to M Excess of after M is ionized: (15.775 – I) eV I is ionization energy of M M has vibrational degree of freedom and will fragment Knowledge of the precise amount of excess of energy given to M Sometimes can selectively ...
... Excess of energy after ionization is 15.755 eV Excess of energy will transfer to M Excess of after M is ionized: (15.775 – I) eV I is ionization energy of M M has vibrational degree of freedom and will fragment Knowledge of the precise amount of excess of energy given to M Sometimes can selectively ...
L-J Chemistry 1 Quiz 25 1 A property that depends on the amount of
... Device to break light down into a spectrum Electrons in the outer level of an atom that can be gained, shared or lost Energy added to an atom when removing an electron Group of atoms held together by covalent bonds A compound composed of four different atoms A polyatomic ion containing oxygen Formul ...
... Device to break light down into a spectrum Electrons in the outer level of an atom that can be gained, shared or lost Energy added to an atom when removing an electron Group of atoms held together by covalent bonds A compound composed of four different atoms A polyatomic ion containing oxygen Formul ...
Mass spectrometry-led catalyst discovery
... capable of studying complex mixtures in solution, capabilities that make it, at first glance, the ideal technique for the direct analysis of homogeneous catalytic reactions. However, a variety of challenges have prevented ESI-MS from being more widely applied in this context, including the invisibil ...
... capable of studying complex mixtures in solution, capabilities that make it, at first glance, the ideal technique for the direct analysis of homogeneous catalytic reactions. However, a variety of challenges have prevented ESI-MS from being more widely applied in this context, including the invisibil ...
Slide 1
... In this laboratory experiment, we will learn: 1. The principle of mass spectrometry (MS) 2. How to perform MS and MS/MS analysis ...
... In this laboratory experiment, we will learn: 1. The principle of mass spectrometry (MS) 2. How to perform MS and MS/MS analysis ...
Ion source

An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.