* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Spectra
Rotational spectroscopy wikipedia , lookup
Rotational–vibrational spectroscopy wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Astronomical spectroscopy wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Isotopic labeling wikipedia , lookup
Two-dimensional nuclear magnetic resonance spectroscopy wikipedia , lookup
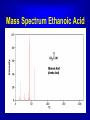
Mass Spectroscopy • Mass spectroscopy is a powerful tool in organic chemistry that can be used to provide information about the molecular formula and structural sub units • When it is combined with techniques of infrared and NMR it can be very useful in identifying unknown compounds The Design of a Mass Spectrometer • The Design of a Mass Spectrometer • The Mass Spectrometer • The mass spectrometer contains a vacuum chamber into which a small amount of a compound is vaporized. The molecules are then bombarded by high energy electrons which cause the molecule to fragment into molecular ions The Mass Spectrometer • The accelerated fragments are passed though a magnetic field that causes them to be deflected. The amount to which a particle isdeflected depends on its molecular mass Mass Spectra A typical molecule may fragment in several different places. The ion formed from the loss of the electron is called the parent ion. The parent ion may fragment into smaller pieces. Each fragment is deflected to a different extent . Hence each peak appears in the Mass Spectrum Peaks in the Mass Spectrum Butane could in four different ways, each of which forms a signal in the mass spectrum Multiplicity of Peaks Carbon has isotopes 13C and 14C as well as 12C. Likewise there are isotopes of hydrogen including 1H, 2H and 3H. Hence a fragment with the formula CH3 has a a predominate mass of 15 but has smaller peaks representing the heavier isotopes of carbon and hydrogen Mass Spectra for Butane Mass Spectrum for Acetone Mass Spectrum Ethanoic Acid Mass Specturm for Toluene Web Site References http://www.chemguide.co.uk/analysis/masspec/mplus.html