physical and chemical change
... Matter is anything that has mass and takes up space. The study of matter and how it changes is called chemistry. Matter can be described in terms of two kinds of properties: physical properties and chemical properties. A physical property is a property of a substance that can be observed without cha ...
... Matter is anything that has mass and takes up space. The study of matter and how it changes is called chemistry. Matter can be described in terms of two kinds of properties: physical properties and chemical properties. A physical property is a property of a substance that can be observed without cha ...
physical and chemical change
... Matter is anything that has mass and takes up space. The study of matter and how it changes is called chemistry. Matter can be described in terms of two kinds of properties: physical properties and chemical properties. A physical property is a property of a substance that can be observed without cha ...
... Matter is anything that has mass and takes up space. The study of matter and how it changes is called chemistry. Matter can be described in terms of two kinds of properties: physical properties and chemical properties. A physical property is a property of a substance that can be observed without cha ...
Introduction to Thermodynamics
... dG = dU + PdV + vdP - TdS - SdT Substitute L1, take constant P,T dG = dQ - TdS This is always less than zero by L2. ...
... dG = dU + PdV + vdP - TdS - SdT Substitute L1, take constant P,T dG = dQ - TdS This is always less than zero by L2. ...
Combustion Chemistry
... Equilibrium Constant • Quantify equilibrium • Consider the generic reaction • Keep in mind that during an equilibration process, reactants/products co-exist ...
... Equilibrium Constant • Quantify equilibrium • Consider the generic reaction • Keep in mind that during an equilibration process, reactants/products co-exist ...
NOTES: 2.1 - Intro to Chemistry
... Life is made up of MATTER! ● MATTER can be defined as anything that has mass and takes up space…this includes individual atoms!! ...
... Life is made up of MATTER! ● MATTER can be defined as anything that has mass and takes up space…this includes individual atoms!! ...
8th Grade Ch. 7 Chemical Reactions Study guide
... ____ 32. According to the law of conservation of mass, how does the mass of the products in a chemical reaction compare to the mass of the reactants? A. There is no relationship. B. The mass of products is sometimes greater. C. The mass of reactants is greater. D. The masses are always equal. ____ 3 ...
... ____ 32. According to the law of conservation of mass, how does the mass of the products in a chemical reaction compare to the mass of the reactants? A. There is no relationship. B. The mass of products is sometimes greater. C. The mass of reactants is greater. D. The masses are always equal. ____ 3 ...
МІНІСТЕРСТВО ОХОРОНИ ЗДОРОВ`Я УКРАЇНИ ХАРКІВСЬКИЙ
... algebraic sum of the enthalpies of formation of the reaction products less the algebraic sum of the enthalpies of formation of the reactants, with account of stoichiometric coefficients (first corollary). The second corollary of Hess’s law relates to heats of combustion (oxidation with oxygen): heat ...
... algebraic sum of the enthalpies of formation of the reaction products less the algebraic sum of the enthalpies of formation of the reactants, with account of stoichiometric coefficients (first corollary). The second corollary of Hess’s law relates to heats of combustion (oxidation with oxygen): heat ...
Syllabus Fundamentals of Physical Chemistry (CHM 3400) Fall 2011
... Outline of course: The chapters in the text will be followed in sequence, with a few omissions. The chapters are short, so we will move through them rapidly (usually not more than one week per chapter). The outline below is ambitious. It is possible that some of the later material will be omitted. T ...
... Outline of course: The chapters in the text will be followed in sequence, with a few omissions. The chapters are short, so we will move through them rapidly (usually not more than one week per chapter). The outline below is ambitious. It is possible that some of the later material will be omitted. T ...
Collision Theory
... Theories of Chemical Kinetics: Collision Theory • Before atoms/molecules/ions can react, they must first collide • An effective collision between two species puts enough energy to break key bonds • The activation energy (Ea) is the minimum energy that must be supplied by collisions to trigger a rea ...
... Theories of Chemical Kinetics: Collision Theory • Before atoms/molecules/ions can react, they must first collide • An effective collision between two species puts enough energy to break key bonds • The activation energy (Ea) is the minimum energy that must be supplied by collisions to trigger a rea ...
What is Weathering
... C. How does iron turn into iron oxide when it is exposed to water? D. If you crumple a piece of paper into a ball, is the change physical or chemical? Physical Why? II. Chemical Changes and Evidence of Chemical Changes A. List three types of evidence of chemical change. B. Compare the second and thi ...
... C. How does iron turn into iron oxide when it is exposed to water? D. If you crumple a piece of paper into a ball, is the change physical or chemical? Physical Why? II. Chemical Changes and Evidence of Chemical Changes A. List three types of evidence of chemical change. B. Compare the second and thi ...
1 Physical Chemistry - Fall 2015 CHEM 410B Room GMCS
... Text: Physical Chemistry, 10th Edition by Peter Atkins and Julio de Paula. The Book Companion Website can be accessed at: http://whfreeman.com/pchem9e Prerequisites: Chem 410A Chemistry 232, 232L, 251 Mathematics 150, 151, 252 Physics 195, 195L, 196, 196L Recommended: Physics 197, 197L The second se ...
... Text: Physical Chemistry, 10th Edition by Peter Atkins and Julio de Paula. The Book Companion Website can be accessed at: http://whfreeman.com/pchem9e Prerequisites: Chem 410A Chemistry 232, 232L, 251 Mathematics 150, 151, 252 Physics 195, 195L, 196, 196L Recommended: Physics 197, 197L The second se ...
CLASS NOTES- Balancing Chemical Equations.pptx
... in the chemical composition of matter • Creates new materials with new properties • AMOUNT of matter does not change! • Chemical equations describe a chemical reaction • Written similar to a mathematical equation/is like a ‘RECIPE’ ...
... in the chemical composition of matter • Creates new materials with new properties • AMOUNT of matter does not change! • Chemical equations describe a chemical reaction • Written similar to a mathematical equation/is like a ‘RECIPE’ ...
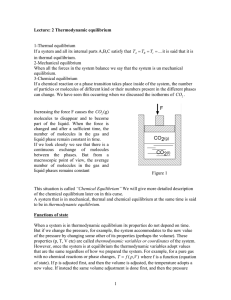
1 Lecture: 2 Thermodynamic equilibrium 1
... of the chemical equilibrium later on in this curse. A system that is in mechanical, thermal and chemical equilibrium at the same time is said to be in thermodynamic equilibrium. Functions of state When a system is in thermodynamic equilibrium its properties do not depend on time. But if we change th ...
... of the chemical equilibrium later on in this curse. A system that is in mechanical, thermal and chemical equilibrium at the same time is said to be in thermodynamic equilibrium. Functions of state When a system is in thermodynamic equilibrium its properties do not depend on time. But if we change th ...
Test 4
... 2. Define the following terms: Spontaneous process A process that will occur without input of energy from a external source. Second law of thermodynamics In any spontaneous process the entropy of the universe always increases. Positional disorder Randomness that comes from the number of different ar ...
... 2. Define the following terms: Spontaneous process A process that will occur without input of energy from a external source. Second law of thermodynamics In any spontaneous process the entropy of the universe always increases. Positional disorder Randomness that comes from the number of different ar ...
10CH301 - Karunya University
... • Students acquire a good understanding of the basic principles of chemical and statistical thermodynamics, application of phase rule to different chemical systems and surface chemistry principles Unit I Chemical Thermodynamics: First law of thermodynamics – Limitation of first law of thermodynamics ...
... • Students acquire a good understanding of the basic principles of chemical and statistical thermodynamics, application of phase rule to different chemical systems and surface chemistry principles Unit I Chemical Thermodynamics: First law of thermodynamics – Limitation of first law of thermodynamics ...
Document
... An equation is balanced when the number of atoms in the reactants are = to the number of atoms in the products ...
... An equation is balanced when the number of atoms in the reactants are = to the number of atoms in the products ...
Bacteria and Virus Research Jigsaw
... An equation is balanced when the number of atoms in the reactants are = to the number of atoms in the products ...
... An equation is balanced when the number of atoms in the reactants are = to the number of atoms in the products ...
CHE 101– Chapter 8 – Study Guide Terms: Products, reactants
... 4. Steps to Complete a Reaction – Be able to fill in the missing products or reactants for chemical reactions a. Identify the type of reaction b. Determine the reaction mechanism c. Determine if the reaction will occur i. Combination, Decomposition, Acid/Base and Combustion reactions are presumed to ...
... 4. Steps to Complete a Reaction – Be able to fill in the missing products or reactants for chemical reactions a. Identify the type of reaction b. Determine the reaction mechanism c. Determine if the reaction will occur i. Combination, Decomposition, Acid/Base and Combustion reactions are presumed to ...
Verdana 30 pt
... behavior with relatively simple and accurate laws, based on measures of volume, pressure and temperature, said state quantities; these, we add the internal energy U of an ideal gas, which is all kinetic and depends only on the temperature. ...
... behavior with relatively simple and accurate laws, based on measures of volume, pressure and temperature, said state quantities; these, we add the internal energy U of an ideal gas, which is all kinetic and depends only on the temperature. ...
Lacture №1. Chemical thermodynamics. The first law of
... If the gas expands, V2 › V1 and work is done by the system and W is negative (we will use sign positive) V2‹ V1 and the work is done on the system and W is positive Note. It may be noted that many books use the opposite sign convention for work!!! (according to the IUPAC recommendation) ...
... If the gas expands, V2 › V1 and work is done by the system and W is negative (we will use sign positive) V2‹ V1 and the work is done on the system and W is positive Note. It may be noted that many books use the opposite sign convention for work!!! (according to the IUPAC recommendation) ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.