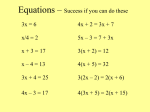* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Review Sheet
Chemical reaction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Electrochemistry wikipedia , lookup
Drug discovery wikipedia , lookup
History of chemistry wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
California Green Chemistry Initiative wikipedia , lookup
Spinodal decomposition wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Safety data sheet wikipedia , lookup
Double layer forces wikipedia , lookup
Al-Shifa pharmaceutical factory wikipedia , lookup
Chemical weapon proliferation wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Chemical plant wikipedia , lookup
Chemical weapon wikipedia , lookup
Chemical industry wikipedia , lookup
Chemical potential wikipedia , lookup
Chemical Corps wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Rate equation wikipedia , lookup
Transition state theory wikipedia , lookup
Stoichiometry wikipedia , lookup
4.02 – Balancing Equations Lesson Review 1. Define reactants. 2. Define products. 3. Define chemical equation. 4. What do each of the following symbols mean in a chemical equation? a. s b. l c. g d. aq 5. Define Law of Conservation of Mass. 6. What do we change in a chemical equation to balance them? 7. What are six tips to balancing equations? 8. Balance the following equations. a. NaCl + F2 NaF + Cl2 b. P + O2 P2O5 c. C3H8 + O2 CO2 + H2O d. AlBr3 + K2SO4 KBr + Al2(SO4)3 4.02 – Balancing Equations Lesson Review Key 1. Define reactants. The substances that are present at the beginning and undergo a chemical reaction. 2. Define products. The new substances that are formed or produced by a chemical reaction. 3. Define chemical equation. A representation, using formulas and symbols, of a chemical reaction. 4. What do each of the following symbols mean in a chemical equation? a. s = solid b. l = liquid c. g = gas d. aq = aqueous 5. Define Law of Conservation of Mass. Matter is neither created nor destroyed during an ordinary physical change or chemical reaction. 6. What do we change in a chemical equation to balance them? coefficients 7. What are six tips to balancing equations? a. Balance each type of element one at a time. b. Whenever you add a coefficient in front of a formula, remember that it affects the number of each atom in that formula. c. Sometimes an element is found in more than one compound on the same side of an equation, which can make it even more challenging to balance. d. There are times when you may have to change a coefficient more than once before the entire equation is balanced, so work with a pencil with an eraser. e. If a polyatomic ion appears on both sides of the equation, you can balance the ion as if it is a single unit. f. Always remember to check and check again! 8. Balance the following equations. a. 2NaCl + F2 2NaF + Cl2 b. 4P + 5O2 2P2O5 c. C3H8 + 5O2 3CO2 + 4H2O d. 2AlBr3 + 3K2SO4 6KBr + Al2(SO4)3