CHM 2045C - State College of Florida
... (5 Credit Hours) (A.A.) Three hours lecture, three hours laboratory per week. Prerequisites: Completion of MAC 1105. Completion of CHM 1025C with a grade of “C” or better or one year of high school college preparatory honors or AP chemistry within last three years. This course meets Area V for the A ...
... (5 Credit Hours) (A.A.) Three hours lecture, three hours laboratory per week. Prerequisites: Completion of MAC 1105. Completion of CHM 1025C with a grade of “C” or better or one year of high school college preparatory honors or AP chemistry within last three years. This course meets Area V for the A ...
The only sure evidence that a chemical reaction has occured is
... 3. A shorter, easier way to show chemical reactions, using symbols instead of words, is called a _____. 4. The substances on the left of the arrow in a chemical equation are the substances you start with called ______. 5. Give an example of a change that is NOT a chemical reaction? 6. How many atoms ...
... 3. A shorter, easier way to show chemical reactions, using symbols instead of words, is called a _____. 4. The substances on the left of the arrow in a chemical equation are the substances you start with called ______. 5. Give an example of a change that is NOT a chemical reaction? 6. How many atoms ...
Unit 5 Chemical Properties and Changes Video Notes A ______ is a
... ________________________ A change that alters the identity of a substance resulting in a new substance or substances with different properties ________________________ Those characteristics that can be observed when a chemical reaction changes the identity of the substance, such as potential to rus ...
... ________________________ A change that alters the identity of a substance resulting in a new substance or substances with different properties ________________________ Those characteristics that can be observed when a chemical reaction changes the identity of the substance, such as potential to rus ...
CHAPTER 8
... freezing, condensation, sublimation, deposition, phase diagrams, crystalline solids. 2. Quantum mechanics: electromagnetic radiation, quantum mechanics, quantum numbers, shape and energy of orbitals, Pauli Exclusion Principle, multi-electron atoms, Aufbau method, valence bond theory, hybridization, ...
... freezing, condensation, sublimation, deposition, phase diagrams, crystalline solids. 2. Quantum mechanics: electromagnetic radiation, quantum mechanics, quantum numbers, shape and energy of orbitals, Pauli Exclusion Principle, multi-electron atoms, Aufbau method, valence bond theory, hybridization, ...
Section 2-4 “Chemical Reactions and Enzymes”
... Energy must be added to break bonds that hold the reactant molecules together. This is called activation energy (Ae). This amount of energy is what “activates” or gets the reaction started. Once the bonds are broken, the atoms are freed up and can make new molecules. When bonds form between the atom ...
... Energy must be added to break bonds that hold the reactant molecules together. This is called activation energy (Ae). This amount of energy is what “activates” or gets the reaction started. Once the bonds are broken, the atoms are freed up and can make new molecules. When bonds form between the atom ...
Biogeochemical cycles and thermodynamics
... in Joules and is a measure of the maximum amount of energy available during transition from one state to the next in an isothermal system at constant pressure. Such conditions typify living systems in thermal equilibrium (especially microorganisms). Gibbs free energy is an extensive state function m ...
... in Joules and is a measure of the maximum amount of energy available during transition from one state to the next in an isothermal system at constant pressure. Such conditions typify living systems in thermal equilibrium (especially microorganisms). Gibbs free energy is an extensive state function m ...
Topic2890 Thermodynamics and Kinetics A given system at
... reaction. In fact the link between the rate of chemical reaction (dξ / dt ) and the affinity for spontaneous change A is intuitively attractive. However while one may monitor the dependence of composition on time, dξ/dt, it is not immediately obvious ∂A how one might estimate the affinity A and ...
... reaction. In fact the link between the rate of chemical reaction (dξ / dt ) and the affinity for spontaneous change A is intuitively attractive. However while one may monitor the dependence of composition on time, dξ/dt, it is not immediately obvious ∂A how one might estimate the affinity A and ...
Standard 4.8
... A They easily form ionic bonds with each other. B They easily form covalent bonds with each other. C They easily combine with atoms of oxygen. D They easily become highly charged ions. ...
... A They easily form ionic bonds with each other. B They easily form covalent bonds with each other. C They easily combine with atoms of oxygen. D They easily become highly charged ions. ...
7th Grade
... When you “pop” the inner pouch, the chemical reaction absorbs heat energy from the surroundings. This is an endothermic reaction. The temperature of the solution falls to about 35 F for 10 to 15 minutes. ...
... When you “pop” the inner pouch, the chemical reaction absorbs heat energy from the surroundings. This is an endothermic reaction. The temperature of the solution falls to about 35 F for 10 to 15 minutes. ...
2.4 Chemical Reactions and Enzymes
... Chemical reactions that release energy often occur on their own, or spontaneously. ...
... Chemical reactions that release energy often occur on their own, or spontaneously. ...
Introduction into thermodynamics Thermodynamic variables
... at constant temperature and pressure. Such conditions can be maintained in a laboratory or attained in nature. Gibbs free energy is therefore of central importance in thermodynamics. It is derived from internal energy and therefore has no absolute scale. Gibbs free energy is de ned as G = U + PV − T ...
... at constant temperature and pressure. Such conditions can be maintained in a laboratory or attained in nature. Gibbs free energy is therefore of central importance in thermodynamics. It is derived from internal energy and therefore has no absolute scale. Gibbs free energy is de ned as G = U + PV − T ...
one way
... Chemical kinetics how fast are chemical reactions? v = velocity of a chemical reaction The velocity of a given chemical reaction ...
... Chemical kinetics how fast are chemical reactions? v = velocity of a chemical reaction The velocity of a given chemical reaction ...
Ch 5.1 The Nature of Chemical Reactions
... Objectives For this Chapter • Understand parts to a chemical equation (reactants, products, yeild sign, double arrow) • Conservation of matter is expressed through balancing chemical equations • Describe difference between endothermic and exothermic reactions ...
... Objectives For this Chapter • Understand parts to a chemical equation (reactants, products, yeild sign, double arrow) • Conservation of matter is expressed through balancing chemical equations • Describe difference between endothermic and exothermic reactions ...
ABCT2772
... a. discriminate different Thermodynamics functions and calculate their values in simple processes b. use the Thermodynamics principles and functions to analysis simple chemical systems and determine the effect of external conditions on their equilibrium positions. c. demonstrate a better understandi ...
... a. discriminate different Thermodynamics functions and calculate their values in simple processes b. use the Thermodynamics principles and functions to analysis simple chemical systems and determine the effect of external conditions on their equilibrium positions. c. demonstrate a better understandi ...
CHM 111: General Physical Chemistry 3 Units
... empirical gas laws, Ideal Gas Equation of State, qualitative treatment of kinetic theory of gases, real gases and deviations from ideal gas laws; liquid, macroscopic properties of liquids, evaporation, vapor pressure and its variation with temperature, boiling point, heat of vaporization, Clausius-C ...
... empirical gas laws, Ideal Gas Equation of State, qualitative treatment of kinetic theory of gases, real gases and deviations from ideal gas laws; liquid, macroscopic properties of liquids, evaporation, vapor pressure and its variation with temperature, boiling point, heat of vaporization, Clausius-C ...
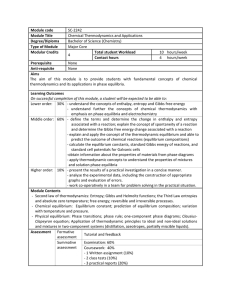
Module code SC-2242 Module Title Chemical Thermodynamics and
... On successful completion of this module, a student will be expected to be able to: Lower order: 30% - understand the concepts of enthalpy, entropy and Gibbs free energy - understand further the concepts of chemical thermodynamics with emphasis on phase equilibria and electrochemistry M ...
... On successful completion of this module, a student will be expected to be able to: Lower order: 30% - understand the concepts of enthalpy, entropy and Gibbs free energy - understand further the concepts of chemical thermodynamics with emphasis on phase equilibria and electrochemistry M ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.