
Matter_and_Change2
... Matter with a uniform and definite composition (also called a pure substance). All samples of a substance have identical physical properties. ...
... Matter with a uniform and definite composition (also called a pure substance). All samples of a substance have identical physical properties. ...
Chapter 2 Study Guides
... 2. What ability allows carbon atoms to form a large number of molecules? ...
... 2. What ability allows carbon atoms to form a large number of molecules? ...
e c n i
... a. Subscript numbers designate how many atoms of each element are present: H2O2 ; 2 Hydrogen atoms and 2 Oxygen atoms are present in this molecule b. W hen no subscript number is shown: it is understood that there is only one atom present: H2O = 2 Hydrogen atoms and only one Oxygen atom are present ...
... a. Subscript numbers designate how many atoms of each element are present: H2O2 ; 2 Hydrogen atoms and 2 Oxygen atoms are present in this molecule b. W hen no subscript number is shown: it is understood that there is only one atom present: H2O = 2 Hydrogen atoms and only one Oxygen atom are present ...
*6th Grade Science-Chapter 5 Study Guide Lesson 5.1: Observing
... A precipitate is a solid that forms from liquids that undergo chemical changes in a chemical reaction. A gas can form from a solid or liquid as a result of chemical changes. A color change can occur as a result of chemical changes. Exothermic reaction- net energy is released from a chemical reaction ...
... A precipitate is a solid that forms from liquids that undergo chemical changes in a chemical reaction. A gas can form from a solid or liquid as a result of chemical changes. A color change can occur as a result of chemical changes. Exothermic reaction- net energy is released from a chemical reaction ...
CYL110
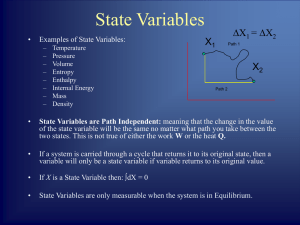
... When a system is at equilibrium, its state is defined entirely by the state variables, and not by the history of the system. The properties of the system can be described by an equation of state which specifies the relationship between these variables. ...
... When a system is at equilibrium, its state is defined entirely by the state variables, and not by the history of the system. The properties of the system can be described by an equation of state which specifies the relationship between these variables. ...
Document
... Treat polyatomic ions as a unit IF it appears on both sides. Leave elements like hydrogen, oxygen, etc. until last. If there is an even # on one side and an odd # on the other, look for the lowest common multiple. ...
... Treat polyatomic ions as a unit IF it appears on both sides. Leave elements like hydrogen, oxygen, etc. until last. If there is an even # on one side and an odd # on the other, look for the lowest common multiple. ...
Course Home - Haldia Institute of Technology
... FT301.2 Ability to understand and introduce the laws of thermodynamics, their implications, and become familiar with their use and applications. FT301.3 Ability to predict intermolecular potential and excess property behavior of multi component systems and ability to determine rate constant of diffe ...
... FT301.2 Ability to understand and introduce the laws of thermodynamics, their implications, and become familiar with their use and applications. FT301.3 Ability to predict intermolecular potential and excess property behavior of multi component systems and ability to determine rate constant of diffe ...
Matter_and_Change2
... Matter with a uniform and definite composition (also called a pure substance). All samples of a substance have identical physical properties. ...
... Matter with a uniform and definite composition (also called a pure substance). All samples of a substance have identical physical properties. ...
PS225 – Heat and thermodynamics
... Heat and work are variables associated with a process. They are not state variables! W pdV area under curve p ...
... Heat and work are variables associated with a process. They are not state variables! W pdV area under curve p ...
Chemical Equations and Reactions
... Hg (mercury) can exist by itself...but, oxygen will need to bond with another oxygen to make O2 (diatomic) To balance the atoms we need to: Put the coefficient of 2 in front of reactant HgO. Put the coefficient of 2 in front the product Hg. ...
... Hg (mercury) can exist by itself...but, oxygen will need to bond with another oxygen to make O2 (diatomic) To balance the atoms we need to: Put the coefficient of 2 in front of reactant HgO. Put the coefficient of 2 in front the product Hg. ...
Matter and Energy
... -atoms found on the reactants side will also be found on the products side. They will be broken apart and rearranged to create new substances. -creates a “Balanced” equation CH4 + 2O2 CO2 + 2H2O ...
... -atoms found on the reactants side will also be found on the products side. They will be broken apart and rearranged to create new substances. -creates a “Balanced” equation CH4 + 2O2 CO2 + 2H2O ...
Chapter 9: Chemical Quantities
... - given moles of a reactant or product you need to be able to use the stoichiometric relationships given in the balanced chemical equation to convert to moles of any other reactant or product -pictorial representations of chemical reactions ...
... - given moles of a reactant or product you need to be able to use the stoichiometric relationships given in the balanced chemical equation to convert to moles of any other reactant or product -pictorial representations of chemical reactions ...
free energy
... substrates for reactions yielding net negative ΔG. Net negative ΔG ensures that reactions proceed in the required direction for continuation of life ...
... substrates for reactions yielding net negative ΔG. Net negative ΔG ensures that reactions proceed in the required direction for continuation of life ...
New Microsoft Office Word Document
... Depends on the path followed from initial to final state of the system ...
... Depends on the path followed from initial to final state of the system ...
Chapter 14 Chemical Reactions
... reacted in a closed container, you can show that the mass before and after the reaction is the same. ...
... reacted in a closed container, you can show that the mass before and after the reaction is the same. ...
CHEMISTRY EXAM 2 REVIEW
... My child completed this review and studied for at least 30 minutes. Define the following chemistry terms: [Chemistry Dictionary] 1. alloy a mixture of metals 2. brittleness the property of matter that is how easily the substance breaks or shatters when force is applied to it. 3. compound a substance ...
... My child completed this review and studied for at least 30 minutes. Define the following chemistry terms: [Chemistry Dictionary] 1. alloy a mixture of metals 2. brittleness the property of matter that is how easily the substance breaks or shatters when force is applied to it. 3. compound a substance ...
1E5 CHEMISTRY [5 credits]
... 10. Carry out basic experimental procedures on aspects of chemical reactions and to appreciate the need for safety and safety procedures in the laboratory. ...
... 10. Carry out basic experimental procedures on aspects of chemical reactions and to appreciate the need for safety and safety procedures in the laboratory. ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.



















![1E5 CHEMISTRY [5 credits]](http://s1.studyres.com/store/data/008628596_1-20bf99494b049c829cfe9aa2d126338b-300x300.png)