CHM_101_ASSIGNMENT_COPY_1_2
... initial concentration is 0.1moldm-3, what is the initial rate in moldm-3s-1. (b) The initial rate of a second order reaction is 5.0×10-7moldm-3s-1, and the initial concentrations of the two reacting substances are each 0.2moldm-3.What is the rate constant in dm3mol-1s-1? (c) The first order rate con ...
... initial concentration is 0.1moldm-3, what is the initial rate in moldm-3s-1. (b) The initial rate of a second order reaction is 5.0×10-7moldm-3s-1, and the initial concentrations of the two reacting substances are each 0.2moldm-3.What is the rate constant in dm3mol-1s-1? (c) The first order rate con ...
Modeling and experimental studies of radical formation in RF discharges with etching gases
... We also measure radical species by setting the extractor at a positive potential and using the electron beam with energies of 15 eV. This electron energy is small enough to prevent c-C5F8 from being dissociated into fragments inside QMS and to measure species only from the plasma. The exception is C ...
... We also measure radical species by setting the extractor at a positive potential and using the electron beam with energies of 15 eV. This electron energy is small enough to prevent c-C5F8 from being dissociated into fragments inside QMS and to measure species only from the plasma. The exception is C ...
Writing Chemical Equations KClO3 O2 (g) + KCl (s) Balancing
... reaction. A formula written above or below the yield symbol indicates that a catalyst is used. In this example Platinum is the catalyst. ...
... reaction. A formula written above or below the yield symbol indicates that a catalyst is used. In this example Platinum is the catalyst. ...
Introduction to Chemical Equations
... • Coefficients change the ratio between reactants and products ...
... • Coefficients change the ratio between reactants and products ...
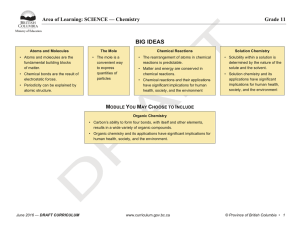
BIG IDEAS - BC Curriculum - Province of British Columbia
... • Formulate physical or mental theoretical models to describe a phenomenon • Communicate scientific ideas, information, and perhaps a suggested course of action, for a specific purpose and audience, constructing evidence-based arguments and using appropriate scientific language, conventions, and rep ...
... • Formulate physical or mental theoretical models to describe a phenomenon • Communicate scientific ideas, information, and perhaps a suggested course of action, for a specific purpose and audience, constructing evidence-based arguments and using appropriate scientific language, conventions, and rep ...
Equilibrium STUDY GUIDE by Keshara Senanayake ---
... Its good to note that even seemingly insoluble solids are actually somewhat soluble in aqueous solutions. Remember the law of mass action, which states that the concentrations of reactants and products at equilibrium are always related to a constant as described by the equilibrium expressions. Solub ...
... Its good to note that even seemingly insoluble solids are actually somewhat soluble in aqueous solutions. Remember the law of mass action, which states that the concentrations of reactants and products at equilibrium are always related to a constant as described by the equilibrium expressions. Solub ...
Basic Concepts
... • can be calculated when equilibrium concentrations, or partial pressures are substituted into the equilibrium constant expression for the reaction. • When equilibrium concentrations are not given the equilibrium concentrations can be obtained from the initial concentrations of the reactants and the ...
... • can be calculated when equilibrium concentrations, or partial pressures are substituted into the equilibrium constant expression for the reaction. • When equilibrium concentrations are not given the equilibrium concentrations can be obtained from the initial concentrations of the reactants and the ...
Chemistry Final Exam Test Yourself I
... one, is it a strong or weak acid? (strong) Which gas law includes pressure, volume, temperature, and the # of moles? ...
... one, is it a strong or weak acid? (strong) Which gas law includes pressure, volume, temperature, and the # of moles? ...
Document
... – One that does not react with components of reaction e.g. argon, helium, neon, usually N2 • Adding inert gas to reaction at fixed V (n and T), increase P of all reactants and products • Since it doesn’t react with anything – No change in concentrations of reactants or products – No net effect on re ...
... – One that does not react with components of reaction e.g. argon, helium, neon, usually N2 • Adding inert gas to reaction at fixed V (n and T), increase P of all reactants and products • Since it doesn’t react with anything – No change in concentrations of reactants or products – No net effect on re ...
The student will
... IV. Relationship between the rate-determining step and the reaction mechanism The student will complete assignments/activities that show they: 1 . Can list the factors that influence the rate of a chemical reaction. 2. Can use experimental data to determine the rate law, determine the order of the r ...
... IV. Relationship between the rate-determining step and the reaction mechanism The student will complete assignments/activities that show they: 1 . Can list the factors that influence the rate of a chemical reaction. 2. Can use experimental data to determine the rate law, determine the order of the r ...
2nd Semester Final Exam Review
... 8. Which 3 of the above solutions will conduct electricity? Why? 9. Will a precipitate form if sodium chloride is mixed with barium hydroxide? If so, what’s the ppt? Use the ppt chart in back of lab manual or on p. 860 fo your textbook. 10. Will a precipitate form if silver nitrate is mixed with cal ...
... 8. Which 3 of the above solutions will conduct electricity? Why? 9. Will a precipitate form if sodium chloride is mixed with barium hydroxide? If so, what’s the ppt? Use the ppt chart in back of lab manual or on p. 860 fo your textbook. 10. Will a precipitate form if silver nitrate is mixed with cal ...
chemical*equations
... “Success'is'not',inal,'failure' is'not'fatal:'it'is'the'courage' to'continue'that'counts.” ''7Winston'Churchill ...
... “Success'is'not',inal,'failure' is'not'fatal:'it'is'the'courage' to'continue'that'counts.” ''7Winston'Churchill ...