Chemical Reactions
... • We use chemical equations to summarize the process of the reactions • Chemical equations should be balanced in order to show that mass is conserved during a reaction • The principle that during chemical reactions, the mass of the products is always equal to the mass of the reactants, is known as t ...
... • We use chemical equations to summarize the process of the reactions • Chemical equations should be balanced in order to show that mass is conserved during a reaction • The principle that during chemical reactions, the mass of the products is always equal to the mass of the reactants, is known as t ...
Part I - American Chemical Society
... (A) Ag3PO4 (Ksp = 1 × 10-16) (B) Ca3(PO4)2 (Ksp = 1 × 10-33) (C) Mg3(PO4)2 (Ksp = 1 × 10-24) (D) AlPO4 (Ksp = 1 × 10-20) 36. Which salt is significantly more soluble in a strong acid than in water? ...
... (A) Ag3PO4 (Ksp = 1 × 10-16) (B) Ca3(PO4)2 (Ksp = 1 × 10-33) (C) Mg3(PO4)2 (Ksp = 1 × 10-24) (D) AlPO4 (Ksp = 1 × 10-20) 36. Which salt is significantly more soluble in a strong acid than in water? ...
K c
... experimental conditions may disturb the balance and shift the equilibrium position so that more or less of the desired product is formed. • In this section we will study 5 factor which can effect chemical equilibrium namely : concentration, pressure, volume, temperature, and catalyst. • Le Châtelier ...
... experimental conditions may disturb the balance and shift the equilibrium position so that more or less of the desired product is formed. • In this section we will study 5 factor which can effect chemical equilibrium namely : concentration, pressure, volume, temperature, and catalyst. • Le Châtelier ...
Free response review
... b. Identify any amphoteric species, other than water, in your equations. c. Assume the amphoteric species you identified in part b is a base. Write an ionic equation for its aqueous ionization and calculate the corresponding Kb. d. Is an aqueous solution of NaHSO3 acidic or basic? Explain your reaso ...
... b. Identify any amphoteric species, other than water, in your equations. c. Assume the amphoteric species you identified in part b is a base. Write an ionic equation for its aqueous ionization and calculate the corresponding Kb. d. Is an aqueous solution of NaHSO3 acidic or basic? Explain your reaso ...
Chemistry 30 - SharpSchool
... two different acids (or bases) can have the same [ ] but have different strengths eg) 1 M CH3COOH(aq) and 1 M HCl(aq) will react in the same way but not to the same degree ...
... two different acids (or bases) can have the same [ ] but have different strengths eg) 1 M CH3COOH(aq) and 1 M HCl(aq) will react in the same way but not to the same degree ...
AP Chemistry Syllabus
... Students interpret molecular spectroscopy data and determine the types of chemical bonds in substances and investigate their electronic structure. Includes infrared, ultraviolet, visible, and photoelectron spectroscopy. Students analyze data, justify the selection of data, use theoretical models to ...
... Students interpret molecular spectroscopy data and determine the types of chemical bonds in substances and investigate their electronic structure. Includes infrared, ultraviolet, visible, and photoelectron spectroscopy. Students analyze data, justify the selection of data, use theoretical models to ...
10-4 Enthalpy (Section 10.6)
... the potential energy stored in the bonds of molecules. Chemists use the change in enthalpy ∆H to measure the heat content of a system (when the pressure is constant). • We define the “system” to be the chemicals and everything else is termed the “surroundings”. • Applying the First Law of Thermodyna ...
... the potential energy stored in the bonds of molecules. Chemists use the change in enthalpy ∆H to measure the heat content of a system (when the pressure is constant). • We define the “system” to be the chemicals and everything else is termed the “surroundings”. • Applying the First Law of Thermodyna ...
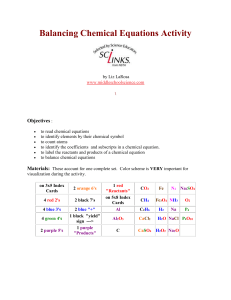
Balancing Chemical Equations Activity by Liz LaRosa www
... The index cards are a bit time consuming to create. I had some students help at lunch time for a few days. Once done, you can laminate them and have them forever! The materials account for one complete set which is good for 2-3 students to use. Print activity cards on card stock instead of making in ...
... The index cards are a bit time consuming to create. I had some students help at lunch time for a few days. Once done, you can laminate them and have them forever! The materials account for one complete set which is good for 2-3 students to use. Print activity cards on card stock instead of making in ...
Equilibrium
... ______________ (the process of dissolving) equals the rate of _______________]. At this instance, ___________ equilibrium has been established. ...
... ______________ (the process of dissolving) equals the rate of _______________]. At this instance, ___________ equilibrium has been established. ...
1 Thermodynamics Reference: Any physical chemistry text book See
... Keq = e-∆G° / RT = 10-∆G°/1.36kcal / mole ∆G° = -(RT)ln Keq = -(2.303RT)log Keq ∆G = ∆G°+ (RT)ln[products] [reactants] ∆G = ∆G°+ (2.303RT) log[products] [reactants] ∆G = ∆G°+ (1.36kcal / mole) log [products] [reactants] ∆G = ∆G°+ (58meV)log [products] ...
... Keq = e-∆G° / RT = 10-∆G°/1.36kcal / mole ∆G° = -(RT)ln Keq = -(2.303RT)log Keq ∆G = ∆G°+ (RT)ln[products] [reactants] ∆G = ∆G°+ (2.303RT) log[products] [reactants] ∆G = ∆G°+ (1.36kcal / mole) log [products] [reactants] ∆G = ∆G°+ (58meV)log [products] ...
A matter of Equilibrium
... Such a state is a dynamical equilibrium – both the forward and backward reactions are proceeding simultaneously but the rates of each balance one another. In other words if we imagine following a particular H atom we would see that it spends some of its time bound in ammonia molecules and some time ...
... Such a state is a dynamical equilibrium – both the forward and backward reactions are proceeding simultaneously but the rates of each balance one another. In other words if we imagine following a particular H atom we would see that it spends some of its time bound in ammonia molecules and some time ...
Please do not remove this page. The periodic table, constants, and
... your name Test Form A the 9-digit ID number given above (rightmost digit blank) You should answer questions for Part II (1 - 15) on the Scantron sheet. You will not have the Scantron returned to you, so if you would like to know what you answered after the exam is returned, circle your answers on th ...
... your name Test Form A the 9-digit ID number given above (rightmost digit blank) You should answer questions for Part II (1 - 15) on the Scantron sheet. You will not have the Scantron returned to you, so if you would like to know what you answered after the exam is returned, circle your answers on th ...
Types of Reactions notes 02 Types of chemical reactions
... NaCl(aq) – means that the chemical is disolved in water. In this case it would be salt dissolved in water. ...
... NaCl(aq) – means that the chemical is disolved in water. In this case it would be salt dissolved in water. ...
Chemical Formulas and Equations
... • Covalent compounds are composed of 2 nonmetals. Many covalent compounds use prefixes that represents a number and tell how many atoms of each element are in the formula. ...
... • Covalent compounds are composed of 2 nonmetals. Many covalent compounds use prefixes that represents a number and tell how many atoms of each element are in the formula. ...