Properties of Systems in Equilibrium - Le
... where Qsp is called the solubility product reaction quotient. Note that, upon mixing two solutions, one containing A+ and the other containing B-, if Qsp < Ksp the system is not at equilibrium, but since no solid AxBy is present the reaction cannot shift to the right and therefore no reaction will b ...
... where Qsp is called the solubility product reaction quotient. Note that, upon mixing two solutions, one containing A+ and the other containing B-, if Qsp < Ksp the system is not at equilibrium, but since no solid AxBy is present the reaction cannot shift to the right and therefore no reaction will b ...
Enthalpy diagram relating the change for a reaction to enthalpies of
... 4. Catalyst speed up rates of reaction without being used up. ...
... 4. Catalyst speed up rates of reaction without being used up. ...
Microwave Spectra, Geometries, and Hyperfine Constants of OCAgX
... They predicted geometries, stretching force constants, dissociation energies, dipole moments, and 35Cl electric field gradients. They also obtained Mulliken populations predicting sizable π-back-donation from the metal to CO, which is not in keeping with the simple rationale for the high CO stretchi ...
... They predicted geometries, stretching force constants, dissociation energies, dipole moments, and 35Cl electric field gradients. They also obtained Mulliken populations predicting sizable π-back-donation from the metal to CO, which is not in keeping with the simple rationale for the high CO stretchi ...
Chapter 3. Stoichiometry
... Convert grams of reactant to moles of reactant (use molar mass), Convert moles of one reactant to moles of other reactants and products (use the stoichiometric ratio from the balanced chemical equation), Convert moles back into grams for desired product (use molar mass). ...
... Convert grams of reactant to moles of reactant (use molar mass), Convert moles of one reactant to moles of other reactants and products (use the stoichiometric ratio from the balanced chemical equation), Convert moles back into grams for desired product (use molar mass). ...
exam review - hrsbstaff.ednet.ns.ca
... *10. Calculate ΔG at 25°C for the following reaction, by first calculating ΔH and ΔS. Once you've found ΔH and ΔS, solve for ΔG using the formula: ΔG = ΔH - T ΔS Also - will this reaction be spontaneous at this temperature? CH3CO2H (l) + 2 O2 (g) → 2 CO2 (g) + 2 H2O (g) ...
... *10. Calculate ΔG at 25°C for the following reaction, by first calculating ΔH and ΔS. Once you've found ΔH and ΔS, solve for ΔG using the formula: ΔG = ΔH - T ΔS Also - will this reaction be spontaneous at this temperature? CH3CO2H (l) + 2 O2 (g) → 2 CO2 (g) + 2 H2O (g) ...
AP Chem unit 13 presentation
... expression raised to the nth power. Knew =Kon K values are customarily written without units. Law of mass action can describe reactions in the solution and gas phase. ...
... expression raised to the nth power. Knew =Kon K values are customarily written without units. Law of mass action can describe reactions in the solution and gas phase. ...
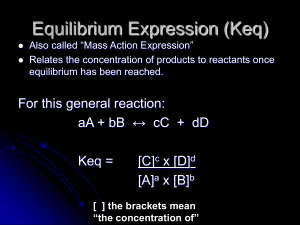
Equilibrium Expression (Keq)
... Ex: Write Keq expression for: NaCl(s) + H2SO4(l) ↔ HCl(g) + NaHSO4(s) Keq = ...
... Ex: Write Keq expression for: NaCl(s) + H2SO4(l) ↔ HCl(g) + NaHSO4(s) Keq = ...
Chemical Reactions
... 2. Find the number of atoms for each element on the left side. Compare those against the number of the atoms of the same element on the right side. 3. Determine where to place coefficients in front of formulas so that the left side has the same number of atoms as the right side for EACH element in o ...
... 2. Find the number of atoms for each element on the left side. Compare those against the number of the atoms of the same element on the right side. 3. Determine where to place coefficients in front of formulas so that the left side has the same number of atoms as the right side for EACH element in o ...
Ch.5
... The number of grams of a substance equivalent to the sum of all its average atomic mass units (amu) is known as the molar mass. ...
... The number of grams of a substance equivalent to the sum of all its average atomic mass units (amu) is known as the molar mass. ...
pH Scale and Concentration Date: Chemistry!
... It has a higher concentration of H 3O+ than OH– and causes litmus to turn blue. It has a higher concentration of OH – than H3O + and causes litmus to turn blue. It has a higher concentration of H 3O+ than OH– and causes methyl orange to turn yellow. It has a higher concentration of OH – than H 3O+ a ...
... It has a higher concentration of H 3O+ than OH– and causes litmus to turn blue. It has a higher concentration of OH – than H3O + and causes litmus to turn blue. It has a higher concentration of H 3O+ than OH– and causes methyl orange to turn yellow. It has a higher concentration of OH – than H 3O+ a ...
VALIDITY OF HENRY`S LAW IN DILUTE SOLUTIONS (l)
... from the ideal case. For thc yalue previously used Kx 10 kt B"x" = 0.01. Results of this calculation are summarizcd in Table n. Table II B',n- ...
... from the ideal case. For thc yalue previously used Kx 10 kt B"x" = 0.01. Results of this calculation are summarizcd in Table n. Table II B',n- ...
Chapter 3 - Bruder Chemistry
... Some students cannot distinguish between the number of moles actually manipulated in the laboratory versus the number of moles required by stoichiometry. Students do not appreciate that the coefficients in an empirical formula are not exact whole numbers because of experimental or round-off errors. ...
... Some students cannot distinguish between the number of moles actually manipulated in the laboratory versus the number of moles required by stoichiometry. Students do not appreciate that the coefficients in an empirical formula are not exact whole numbers because of experimental or round-off errors. ...
Thermochemical Approaches to Neutralization Reactions between
... Reaction enthalpy for the formation of water from aqueous hydrogen ion and hydroxide ion. Assuming simply the reaction is composed of the acid dissociation of penolic compounds in aqueous solutions and the formation of water from aqueous hydrogen ion and hydroxide ion, the value of ∆rH is estimated ...
... Reaction enthalpy for the formation of water from aqueous hydrogen ion and hydroxide ion. Assuming simply the reaction is composed of the acid dissociation of penolic compounds in aqueous solutions and the formation of water from aqueous hydrogen ion and hydroxide ion, the value of ∆rH is estimated ...
K eq
... 1. Each student wads up two paper wads. 2. You must start and stop as the timekeeper says. 3. Throw only one paper wad at a time. 4. If a paper wad lands next to you, you must throw it back. ...
... 1. Each student wads up two paper wads. 2. You must start and stop as the timekeeper says. 3. Throw only one paper wad at a time. 4. If a paper wad lands next to you, you must throw it back. ...
Document
... Students will be able to describe the difference between a strong and weak acid or base Students will be able to articulate the conceptual and mathematical relationships between pH, Ka and Keq Students will be able to describe the solution of the equation for Ka given initial conditions Exerci ...
... Students will be able to describe the difference between a strong and weak acid or base Students will be able to articulate the conceptual and mathematical relationships between pH, Ka and Keq Students will be able to describe the solution of the equation for Ka given initial conditions Exerci ...
Lecture Notes V
... To describe the chemical energy released when fuel reacts with air to form products, chemical species in the reactants and products and their states are specified. Heat of vaporization of liquid fuels and heat of pyrolysis of solid fuels is small compared with the chemical energy relased by combusti ...
... To describe the chemical energy released when fuel reacts with air to form products, chemical species in the reactants and products and their states are specified. Heat of vaporization of liquid fuels and heat of pyrolysis of solid fuels is small compared with the chemical energy relased by combusti ...
Balancing Equations
... The overall change in energy for certain rxns can be found on Ref. Table I ...
... The overall change in energy for certain rxns can be found on Ref. Table I ...
Chemistry Lab 2010
... b = path length (cm) c = concentration (M) ε = Molar absorbtivity (constant, units M-1 cm-1) Lambert-Beer or Beer’s Law Plot: Provided all absorbance measurements are made on the same spectrophotometer with the same cell, a graph of absorbance vs. concentration will be linear. Example: Copper (II) i ...
... b = path length (cm) c = concentration (M) ε = Molar absorbtivity (constant, units M-1 cm-1) Lambert-Beer or Beer’s Law Plot: Provided all absorbance measurements are made on the same spectrophotometer with the same cell, a graph of absorbance vs. concentration will be linear. Example: Copper (II) i ...
KEY Final Exam Review - Iowa State University
... Note that 16 and 3 have no common factors except 1, so both 16 and 3 had to be used to obtain the lowest common multiple of 48 for the number of electrons. 4) Add: 24H2S + 16H+ + 16NO3¯ ---> 3S8 + 16NO + 32H2O Comment: removing a factor of 8 does look tempting, doesn't it? However, the three in fron ...
... Note that 16 and 3 have no common factors except 1, so both 16 and 3 had to be used to obtain the lowest common multiple of 48 for the number of electrons. 4) Add: 24H2S + 16H+ + 16NO3¯ ---> 3S8 + 16NO + 32H2O Comment: removing a factor of 8 does look tempting, doesn't it? However, the three in fron ...
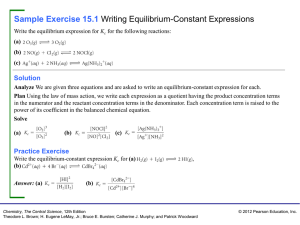
Sample Exercise 15.1 Writing Equilibrium
... Sample Exercise 15.7 Analyzing a Heterogeneous Equilibrium Each of these mixtures was placed in a closed container and allowed to stand: (a) CaCO3(s) (b) CaO(s) and CO2(g) at a pressure greater than the value of Kp (c) CaCO3(s) and CO2(g) at a pressure greater than the value of Kp (d) CaCO3(s) and ...
... Sample Exercise 15.7 Analyzing a Heterogeneous Equilibrium Each of these mixtures was placed in a closed container and allowed to stand: (a) CaCO3(s) (b) CaO(s) and CO2(g) at a pressure greater than the value of Kp (c) CaCO3(s) and CO2(g) at a pressure greater than the value of Kp (d) CaCO3(s) and ...
Properties_problems 5
... where Ev is defined as the energy change upon isothermal vaporization of the saturated liquid to the ideal gas state at infinite dilution and Vi is the molar volume of the liquid. The solubility parameter of a polymer has to be determined indirectly or calculated by group-contribution methods. Calc ...
... where Ev is defined as the energy change upon isothermal vaporization of the saturated liquid to the ideal gas state at infinite dilution and Vi is the molar volume of the liquid. The solubility parameter of a polymer has to be determined indirectly or calculated by group-contribution methods. Calc ...