* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download constant pressure
Determination of equilibrium constants wikipedia , lookup
Van der Waals equation wikipedia , lookup
Thermal radiation wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Transition state theory wikipedia , lookup
Glass transition wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Heat transfer wikipedia , lookup
Heat transfer physics wikipedia , lookup
Temperature wikipedia , lookup
Thermodynamics wikipedia , lookup
Heat equation wikipedia , lookup
Thermal expansion wikipedia , lookup
Thermal conduction wikipedia , lookup
Thermoregulation wikipedia , lookup
Calorimetry wikipedia , lookup
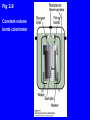
Peter Atkins • Julio de Paula Atkins’ Physical Chemistry Eighth Edition Chapter 2 The First Law Copyright © 2006 by Peter Atkins and Julio de Paula Heat transactions In general: dU = dq + dwexp + dwe where dwe ≡ extra work in addition to expansion At ΔV = 0 and no additional work: dU = dqV For a measurable change: ΔU = qV • Implies that ΔU can be obtained from measurement of heat • Bomb calorimeter used to determine qV and, hence, ΔU Fig 2.9 Constant-volume bomb calorimeter Fig 2.10 Change in internal energy as function of temperature The heat capacity at constant volume: U CV T V Slope = (∂U/∂T)V Fig 2.11 Change in internal energy as a function of temperature and volume • U(T,V), so we hold one variable (V) constant, and take the ‘partial derivative’ with respect to the other (T). δU CV δT V Fig 2.12 At constant volume: dU = dq If system can change volume, dU ≠ dq • Some heat into the system is converted to work • ∴ dU < dq • Constant pressure processes much more common than constant volume processes If CV is assumed to be constant with temperature for macroscopic changes: ΔU = CV ΔT qV = CV ΔT or: Enthalpy ≡ heat flow under constant pressure H ≡ U + PV ΔH = ΔU + PΔV ΔH = ΔU + ΔngRT Fig 2.14 Plot of enthalpy as a function of temperature The heat capacity at constant pressure: CP = (∂H/∂T)P H CP T P Cp > Cv Cp – Cv = nR CV = (∂U/∂T)V Variation of enthalpy with temperature H CP T P If CP is assumed to be constant with temperature for macroscopic changes: ΔH = CP ΔT qP = CP ΔT or: If ΔT ≥ 50 oC, use empirical expression, e.g.: CP,m a bT c T2 with empirical parameters from Table 2.2 Adiabatic Changes • Consider change of state: Ti, Vi → Tf, Vf • Internal energy is a state function ∴ change can be considered in two steps Fig 2.17 Variation of temperature as a perfect gas is expanded reversibly and adiabatically: Vf Tfc ViTic where: c CV ,m R Fig 2.17 Fig 2.18 (a) Variation of pressure with volume in a reversible adiabatic expansion Pf Vfγ Pi Viγ where the heat capacity ratio: γ CP,m C V ,m Fig 2.18 (b) • Pressure declines more steeply for an adiabat than for an isotherm • Temperature decreases in an adiabatic expansion