* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Equilibrium STUDY GUIDE by Keshara Senanayake ---
Multi-state modeling of biomolecules wikipedia , lookup
Asymmetric induction wikipedia , lookup
Supramolecular catalysis wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Double layer forces wikipedia , lookup
Thermomechanical analysis wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Electrolysis of water wikipedia , lookup
Acid–base reaction wikipedia , lookup
Marcus theory wikipedia , lookup
Process chemistry wikipedia , lookup
Crystallization wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Thermodynamics wikipedia , lookup
Electrochemistry wikipedia , lookup
Hydroformylation wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Chemical reaction wikipedia , lookup
George S. Hammond wikipedia , lookup
Click chemistry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Rate equation wikipedia , lookup
Stoichiometry wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Transition state theory wikipedia , lookup
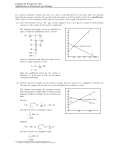
Equilibrium STUDY GUIDE by Keshara Senanayake ---Textbook --> Class notes *Examples are underlines Chapter 16! Equilibrium exists when two opposing processes occur at the same rate. Even though changes are occuring, there is no overall change. ---> Equilibria is the plural of equilibrium A perfect example of equilibrium: When a liquid is placed in a closed container, some molecules leave the liquid state and enter the gaseous state forming a vapor above the liquid which forms a vapor above the liquid. While at the same time, some molecules in the gaseous state strike the surface of the liquid and return to the liquid state. THESE ARE OPPOSING PROCESSES >> When the rate at which molecules escape from the liquid equals the rate at which molecules return to the liquid state equilibrium is reached. A chemical reaction in which the products can regenerate the original reactants is called a reversible reaction. **In theory all chemical reactions are reversible reactions. Some reactions are reversible on their own, others are reversible under certain conditions When a reaction is at equilibrium instead of an arrow you use two half arrows. As we learned previously the reaction rate depends on the concentration of the substances in the reaction, the temperature at which the reaction takes place, and (for gases) the pressure at which the reaction takes place. Reaction rate is PROPORTIONAL to the concentration. Reaction rates are faster when the concentration of the reactants increase. This idea comes into play when during a chemical reaction when the concentration of the reactants decreases as the reactants are converted into products. (So the concentration of the products increase.) For a equilibrium reaction after the reactants are done, the products then decrease (converted back to reactant) and so reactants increase. Each of the two reactions proceeds at its own rate. Because the concentration of each substance changes, the rate of reaction changes throughout the reaction. The rate of the forward reaction decreases from its original rate as the concentration of the products decreases. The rate of the reverse reaction increases from 0 as the concentration of the products increases. Eventually the reaction reached chemical equilibrium at which the rate of the forward reaction is equal to the rate of the reverse reaction Example: 2NO2 (g) N2O4 (g) (remember the experiment Mrs.Arora did in class involving the two tubes of gas, that went from colorless to brown. Well NO2 is the brown gas, N2O4 is the colorless one. This as seen is an equilibrium reaction) (if confused refer to the last paragraph on pg 537) Chemical equilibrium is the state in concentrations of reactants and products remain constant with time because the rate at which they are formed in each reaction equals the rate at which they are formed in each reaction equals the rate at which they are consumed in the opposite reaction. Chemical equilibrium occurs when opposing reactions are proceeding at equal rates. Here's two diagrams to help! The top graph shows the concentration of A decreasing to a constant level and the concentration of B increasing to a constant level as a reversible reaction proceeds. The bottom graph shows what happens to the reaction rates as concentrations changes. The rate of the forward reaction decreases to a constant rate while the rate of the reverse reaction increases at the same constant rate. To denote the concentration of each substance use square brackets. SO the concentration of substance A is [A] and the concentration of substance B is [B] As A reacts to form B, [A] decreases and [B] increases. As [A] decreases the rate of the forward reaction decreases while as [B] increases the rate of the reverse reaction increases. Eventually the reaction does reach equilibrium, the reverse and forward reactions are proceeding at the same rate and the concentrations of A and B are constant. Chemical equilibrium is a dynamic process (changing) (substance A changed to B thenn back and continues to do so. Both processes occur at the same rate and the net change of concentration is 0.) Known as Dynamic Equilibrium ---------------The Equilibrium Constant The law of mass action expresses the relative concentration of reactants and products at equilibrium in terms of a quantity called the equilibrium constant. Look at the general equation: aA + bB cC + dD In this equation a,b,c and d are coefficients for substances A,B,C, and D. The equilibrium expression for this reaction is: Keq = [C]^c [D]^d / [A]^a [B]^b Keq is the equilibrium constant. The equilibrium constant is equal to the ration of the products concentration (raised to the power of their coefficients) to the reactants (raised to the power of their coefficients) REMEMBER square brackets mean concentration. Concentration of the products of the forward reaction are found in the numerator, and the concentration of the reactants of the forward reaction are found in the denominator. This ratio is always constant given a reaction regardless of initial concentrations at any given temperature. According to the law of chemical equilibrium, every reversible reaction proceeds to an equilibrium state that has a specific ratio of the concentrations of reactants and products expressed in Keq The value of the equilibrium constant DOES NOT depend on the initial concentration of the reactants and products. Regardless of the initial concentrations each reaction will establish equilibrium, and that equilibrium can be described by the same equilibrium constant. You can see that the equilibrium state can be attained beginning with either product or reactant -- so the equilibrium state can be reached from either direction the forward or reverse reaction. Each set of equilibrium concentrations is called an equilibrium position. THE EQUILIBRIUM POSITION DEPENDS ON THE INITIAL CONCENTRATIONS but the equilibrium constant does not. There are an infinite number of equilibrium positions, but there is only one equilibrium constant. pg 542 - 543 in the textbook gives specific examples Equilibrium constant does not tell you anything about time it take to reach equilibrium but it does tell you important information about the mixture of reactants and products at equilibrium. The equilibrium constant is a measure of the extent to which a reaction proceeds to completion. ----> Shorthand notation for showing that the equilibrium constant is much greater than 1 is Keq >> 1 (>> just means its just significantly greater than) *Note: if the equilibrium constant is very large, the numerator of the equilibrium expression must be larger than the denominator, which means the equilibrium concentration of the products must be much greater than that of the reactants. At equilibrium then, this reaction system consists mainly of products and is therefore considered to proceed to completion. (it "lies to the right" toward product side) ----> Shorthand notation for showing that the equilibrium constant is less than 1 is Keq << 1 (<< significantly less than) *Note: if the equilibrium constant is very small it indicates that the denominator of the equilibrium expression must be much larger than the numerator. At equilibrium this reaction system would consist of reactants mostly and almost no products. Equilibrium in this case "lies to the left" towards reactant side. If Keq is = 1 considerable concentrations of both reactants and products are present at equilibrium. -Equilibrium conditions for reactions in which all the reactants and products are in the same state are called homogeneous equilibria. -Equilibrium conditions for reactions that involve substances in more than one state are called heterogeneous equilibria Concentrations are expressed in molarity (which is kilograms of solute dissolves in liters of solution) The concentration of a pure substance (liquid or solid) is its density divided by its molar mass. The density of a pure liquid or solid is constant and changes very little with temperature. SO the concentration of a pure solid or liquid goes not change during a reaction. SO when writing an equilibrium expression DO NOT INCLUDE AND LIQUIDS OR SOLIDS The equilibrium expression for NH4Cl(s) HH3(g) + HCl (g) is Keq = [NH3][HCl] *please note though to reach equilibrium these substances (solids and liquids) are still important since their concentration don’t change their not included in the equilibrium constant The reaction quotient (Q) is used to determine if a reaction is at equilibrium. The reaction quotient is calculated much like the equilibrium constant except that the concentrations that exist at the time the measurement is taken, not the equilibrium concentrations are inserted into the equilibrium expression. If the reaction is at equilibrium Q would equal Keq But if: Q is less than Keq (Q < Keq), the denominator of the reaction quotient expression is too large and the numerator is too small. This means that at the time of measurement there is too much of the reactants and too little of the products. The reaction will consume reactants and form the products to reach equilibrium, Thus the reaction will proceed to the right, in the direction of the products. If Q is greater than Keq (Q > Keq) the denominator of the reaction quotient expression is to small and the numerator is too large. This means that at the time of measurement there is too much of the products and too little of the reactants. For the system to reach equilibrium reactants must be formed and products must be consumed. The reaction will proceed to the left, in the direction of the reactants. If Q is equal to Keq (Q = Keq) the system is at equilibrium and no shift in direction will occur --Le Chatelier's principle states that if a change in conditions is imposed on a system at equilibrium, the equilibrium position will shift in the direction that tends to reduce that change in conditions ----------> Basically a reaction system will shift in forward or reverse direction to "undo" a change Changes in concentration shifts the equilibrium position. If more of a particular substance is added to a reaction at equilibrium, the concentration of the substance increases. The reaction will return to equilibrium by consuming some of the added substance. If a substance is removed its concentration decreases. The reaction will return to equilibrium by producing more of the substance that was removed. REMEMBER according to Le Chatelier's principle the reaction will run in the direction that will counter the disturbance. Here's an example: I'll use the equation 2 NO2 N2O4 If more NO2 is injected to the mixture the reaction will run counter to the disturbance and try to consume the extra NO2, so the reaction will run to the right. If more N2O4 is added the reaction will turn to the left consuming the extra N2O4 Why is this so? ---> you can go back to the information of reaction quotient to prove this Since the Q for this reaction is Q = [N2O4]/[NO2]^2 --> And if you increase NO2 the denominator increases resulting in a lower Q value, so Q < Keq and because of this in order to reach equilibrium now it must shift to the right. The opposite happens when you increase the numerator. (then Q > Keq and the reaction shifts to the left) Changes in Pressure For some gaseous equilibrium systems, the reaction can be shifted forward or backward by changing the pressure. One way of changing the pressure of a system is by changing the volume of the container. If the total pressure of a system is increased the system will shift to reduce the pressure by proceeding in the direction that produces fewer molecules (it'll go to the side with the fewer number of moles) Also note DECREASE VOLUME = INCREASE PRESSURE INCREASE VOLUME = DECREASE PRESSURE For example: 2 NO2 N2O4 Increase the pressure it'll go to the right side. Decrease the volume it'll go to the right side. Decrease the pressure it'll go the left side. Increase the volume it'll go to the left side. PLEASE NOTE THOUGH! If its like NH4Cl(s) NH3(g) + HCl(g) 0 moles of gas on the left side produces 2 moles of gas on the right. The reaction will turn from right to left in order to reduce the number of moles of gas produced. ALSO NOTE! If both sides have equal # of moles of gas a shift in either direction will not reduce the pressure. So a increase in pressure has no effect on the equilibrium situation. So an equilibrium reaction that has the same number of moles of gas on both sides of the equation will not be affected by the changes in pressure. Changes in temperature Remember temperature is very important, the value of the equilibrium constant depends on temperature. Increasing the temperature causes some chemical reactions to proceed more completely into products increasing the value of the equilibrium constant. But increasing the temperature causes other chemical reactions to proceed less completely lowering the equilibrium constant. I'll use an example to explain since it's easier. Straight out of the textbook: H2(g) + I2(g) 2HI(g) + heat --> The equilibrium constant for this reaction is 54.5 at 400 Celsius and 45.9 at 490 Celsius. REMEMBER the equilibrium constant is the measure of the extent to which a reaction proceeds. You can thus conclude that RASING THE REMPERATURE FOR THIS REACTION PROCEED LESS COMPLETELY TO PRODUCTS Lowering the temperature would produce more HI -------> We use Le Chaterlier's principle to understand. The reaction above GIVES off heat and is exothermic. In the reversible reaction its endothermic. SO if heat is added to the system the reaction tends (to reestablish equilibrium) by consuming the additional heat through reverse (endothermic reaction). SO as heat is ADDED to this reaction will go to the left side (SINCE HEAT APPEARS ON THE RIGHT) For the reaction heat + NH4Cl (s) NH3(g) + HCl (g) Raising the temperature will cause the reaction to go to the right.. Changing the temperature causes a reaction to reestablish equilibrium by effectively changing the value of the equilibrium constant. *while concentration and pressure effect equilibrium position* HABER PROCESS--> Fritz Haber (1868-1934) wanted to produce more ammonia from a reaction of nitrogen and hydrogen. In the Haber process ammonia is continuously removed (note the reaction is: N2(g) + 3H2 2NH3(g) + heat) so from out previous knowledge (or from above) the reaction shifts to the right to produce more ammonia and restore equilibrium. The pressure is also changed and the temperature is increased (to changed the equilibrium constant) BUT as we know increasing the temperature causes the reverse reaction so we need even high pressures to compensate. CHAPTER 17! Yes! So excited! I get to type more! The process in which an ionic solid dissolves in a polar liquid is called dissolution. (For example then NaCl, or simple table salt is dissolved in water the Cl^- ions get attracted to the positive H+ ion and the Na^+ ion gets attracted to the O^- ions) The dissolution of AgCl in water is: AgCl(s) --> Ag+ (aq) + Cl (aq) = aqueous solution But say if an Ag+ or a Cl- ion collides back to the surface of a Agcl(s) the ion may lose its shell of water molecule and rejoin the solid state. The process in which ion leaves a solution and regenerate an ionic solid is called precipitation Note! Dissolution and precipitation are OPPOSITE processes The equation for this is AgCl(s) <----- Ag+ (aq) + Cl - (aq) All of this is factored back into solubility (which is the maximum quantity of that substance that call dissolve in a given solvent) at some point no additional AgCl can dissolve because the solution is saturated. Although ions continue to dissolve other ions begin to precipitate out of the solution. WHEN DISSOLUTION AND PRECIPITATION OCCUR AT THE SAME RATE ([Ag+] and [Cl-] have constant values) and the solution is said to have attained SOLUBILITY EQUILIBRIUM because the saturated solution of ions and remaining solid are in chemical equilibrium A solubility equilibrium equation can be written to incorporate both dissolution and precipitation. Example for silver chloride its AgCl(s) Ag+ (aq) + Cl - (aq) PLEASE NOTE when writing dissolution equations take the coefficient and charge into account Example: CaCl2(s) ----> Ca^2+ (aq) = 2 Cl It must be broken up to their ions in the chemical formula. Both sides must be electrically neutral. THE SOLUBILITY PRODUCT Its good to note that even seemingly insoluble solids are actually somewhat soluble in aqueous solutions. Remember the law of mass action, which states that the concentrations of reactants and products at equilibrium are always related to a constant as described by the equilibrium expressions. Solubility equilibria can also be described by equilibrium expressions. Example: AgCl(s) Ag+ (aq) + Cl- (aq) The Ksp is [Ag+][Cl-] (you don't include the AgCl since its a pure solid) Ksp is the solubility product constant, or also known as SOLUBILITY PRODUCT because it incorporates a constant value of the solid in solution. The solubility product expression is used for aqueous solutions of dissociated ions. The value of Ksp for a substance can be calculated from the concentration of ions at equilibrium. Example: Given AgCl(s) is added to pure water and allowed to come to equilibrium with a solution of its ions at 25 Celsius. Say at equilibrium [Ag+] = 1.3 X 10^-5 M and [Cl-] = 1.3 X 10^-5 M inserting these values you get Ksp = [Ag+][Cl-] Ksp = (1.3 X 10^-5 M)(1.3 X 10^-5 M ) Ksp = 1.7 X 10^-10 No units are usually assigned NOTE! Its important to know the difference between the solubility of a given solid and its solubility product. The solubility is an equilibrium position and has an INFINITE NUMBER OF POSSIBLE VALUES at given temperatures depending on what other solutes are present in the solution. The solubility product on the other hand is an equilibrium constant and has only one value for a given solid at a given temperature. A small Ksp value indicates that a substance is not very soluble in water. You can also use the value of Ksp to predict the equilibrium concentrations of ions in a saturated solution. Example: CaSO4(s) is placed in pure water at 25 Celsius. What are the concentrations of Ca^2+ and SO4^2- in the solution? Alrighty! The expression given is: CaSO4(s) Ca^2+ (aq) + SO4^2- (aq) ---------> Which is Ksp = [Ca^+2][SO4^-2] We know from the balanced equation above the number of calcium ions is equal to the number of sulfate ions produced when calcium sulfate dissolves. You can represent each concentration by "x" SO Ksp = x * x = x^2 Since you're given the Ksp and its equal to 2.5 X 10^-5 You just plug it in 2.4 X 10^-5 = x^2 square root of (2.4 X 10^-5 ) = x 4.9 X 10^-3 M = x Since both [Ca^2+] and [SO4^-2] ions have equal concentrations they both are 4.9 X 10^-3 M Precipitates Remember when a solution contains more particles (or dissolved ions) than it can hold its known to be saturated. A supersaturated solution is an unstable, no equilibrium state achieved by manipulating conditions such as temperature. Thus a precipitate will form in a supersaturated solution. You can use Ksp for a dissolved substance and the existing concentration of ions to see if a solution is supersaturated. You can insert in the actual concentrations measured at a given point into the equilibrium expression and compare the result with the Ksp. THIS IS THE REACTION QUOTIENT (Q) as we used before. Its also known as the ion product. The ion product can be compared with the solubility product to determine if an aqueous solution of ions is supersaturated and will form a precipitate. IF Q is > than Ksp the numerator is too large relative to the value of the denominator. Thus the concentration of ions is too high and the solution is supersaturated. To attain equilibrium the ions must precipitate from the solution lower the concentration of the ions to their equilibrium values. If Q is < than Ksp the numerator is to small relative to the denominator and the compound will continue to dissolve until ion concentrations reach equilibrium levels. If Q = Ksp Solution is just saturated Let me give you an example! PbCl2(s) Pb^2= (aq) + 2Cl^- (aq) 0.010 mol of PbCl2(s) is dissolved in 150 mL of hot water and the solution is cooled slowly to 25 Celsius The volume of the final solution is 150 m. Is the solution supersaturated? First solve it. Then turn to the next page. 1) Write it down PbCl2(s) Pb^2= (aq) + 2Cl^- (aq) 2) Since the equation shows each mole of PbCl2(s) will produce 1 mole of Pb^2+ and 2 moles of Cl^- in the solution you can conclude 0.010 mole of PbCl2 will produce 0.010 moles of Pb^2+ and 0.020 moles of Cl^- (since you know TWO moles of Cl are produced) 3) REMEMBER concentration of each ion is then the number of moles divided by the number of liters of solution. SO! [PB^2+] = (0.010 mol Pb^2+ / 150 mL) X 1000mL/1L = 0.067 mol/L [CL-] = (0.020 mol Cl- / 150ml) X 1000mL/1L) = 0.13 mol/L 4) Insert the concentrations back into the solubility product expression. Ksp = [Pb^2+][Cl-]^2 Q = (0.067M)(0.13M)^2 The solubility product of PbCl2 is 1.6 X 10^-5 (you had to find it on that worksheet, its given). SO Q is greater than Ksp so the solution is supersaturated. To attain equilibrium the PbCl(s) must precipitate from the solution. Precipitation Reactions A reaction in which two solutions are mixed and a precipitate forms is called a precipitate reaction. When a formula of an ionic compound is written followed by (aq) it is actually being described as a solution of its ions. Thus when two solutions are mixed, two sets of ions are being mixed. The final solution contains all of the ions from each of the individual solutions. The precipitate that forms must be a combination of those ions. Then the question arises, Which two ions combined to form the precipitate? You can use the solubility rules to help you. Remember, not so soluble (says in textbook as sparingly which means the same thing) substances dissolve so slightly in water that a small concentration of ions saturates the solution. Not so soluble/ insoluble are used as similar terms (samething when dealing with these kinds of things( THE SOLUBILITY rules classify a substance as not so soluble/insoluble if only 0.01 mole or less dissolves in a liter of water. Dissociating into ions only slightly in solution, sparingly soluble substance will usually precipitate in a precipitation reaction. Example: AgNO3(aq) + KBr (aq) --> AgBr (s) + KNO3(aq) Since the NO3- is soluble it will dissolve. WHILE Ag+ are generally insoluble thus KNO3 must dissociate into ions in solution while AgBr must be the insoluble precipitate NOTICE a precipitate reaction is an example of a double replacement reaction. *Note not all combinations of compounds form double replacement reactions *if the products have less energy and are therefore more stable than the reactants the reaction tends to proceed to completion In an aqueous solution an insoluble substance is more stable as a solid than as dissociated ions in solution. An insoluble substance formed in a solution is a precipitate. Other common driving forces in double replacement reactions are formation of water and the formation of a gas. *because sparingly soluble substances precipitate in a double replacement reaction you can use the solubility rules to predict whether a particular double replacement reaction is likely to proceed. NOTE SOLUBILITY RULES ARE IN THE REFERENCE TABLE (more in depth on this on pg 574-576) but to note even if a precipitation reaction can proceed it still does not always proceed, Whether or not a precipitate actually forms in a double replacement reaction depends on the concentration of the dissolved ions after the solution is mixed. If the final solution is dilute -- not precipitate. If the ion product exxeeds the solubility product precipitate will form. NET IONIC EQUATION An equation that shows all soluble ionic substances as ions is called a complete ionic equation. A complete ionic equation is found by writing each original solution as the sum of its constituent ions. Complete ionic equations are balanced just like any other chemical equation. For example: Cu(NO3)2) forms Cu^2+ and NO3^- ions in solution and NaOH forms Na^3+ and OH^- ions in solution. This the complete ionic equation is: Cu^2+ (aq) + 2NO3^-(aq) + 2 Na^+ (aq) + 2OH^- (aq) --> Cu(OH)2 (s) + 2Na^+ (aq) + 2NO3^- (aq) Notice! The total charge on each side of the equation is zero. The point of writing it is to notice that the hydroxide ions and the copper (II) ions undergo a chemical change. The Sodium ions and nitrate ions are unchanged! Ions that do not take part in a chemical reaction and are found in solution both before and after the reaction are called SPECTATOR IONS because they "watch" while their more reactive partners undergo chemical change. If we remove the spectator ions we get a simpler equation called the NET IONIC EQUATION which is Cu^2+ (aq) + 2OH^- (aq) --> Cu(OH)2 (s) A net ionic equation includes only those compounds and ions that undergo a chemical change in a reaction in an aqueous solution. Net ionic equations are frequently used to describe precipitation reactions. COMMON- ION EFFECT The common-ion effect is a shift in equilibrium that occurs because the concentration of an ion that is part of the equilibrium is changed. ~ Where the addition of a ion common to two solutes brings about precipitation (called common-ion effect) --> The common-ion effect also lowers the solubility of a sparingly soluble substance Example: CaSO4(s) Ca^2+ (aq) + SO4^2- (aq) Suppose Sodium sulfate (Na2SO4) is added to a saturated solution of calcium sulfate. What will happen to the solubility equilibrium for CaSO4? >> Remember! as NaSO4 dissolves Na+ and SO4^2- ions are added to the solution. BECAUSE BOTH FORM SULFATE IONS, THE SULFATE ION IS CALLED A COMMON ION. While the sodium ions are just being introduced into the solution, the sulfate ions are added to the sulfate ions already presented in the solution from the calcium sulfate. THUS the concentration of sulfate ions is increased beyond equilibrium level. Based on Le Chatelier's principles enforced earlier, it predicts that if the concentration of a substance in a reaction at equilibrium is changed, the reaction will proceed in the direction that minimizes the change. CaSO4(s) <--- Ca^2+ (aq) + SO4^2- (aq) SO according to Le Chatelier's principle this solution will return to equilibrium by removing sulfate ions which is done by precipitating CaSO4 NOTES --------- Reversible reaction: reaction in which the products can react under suitable conditions to product the original products A + B --> C + D (irreversible reaction) A+B C + D (reversible reaction) Characteristics: - two equal and opposite processes should occur at the same rate (reversible reaction) - rate of forward reaction = rate of reverse reaction (Rf = Rr) - concentration of all the reactants and products remain constant - changes are observed if a system is subjected to stress (temperature, pressure, and concentration) otherwise no changes are observed Types of Equilibria 1) Phase equilibria (only phase change) rate of evaporation = rate of condensation 2) Solution equilibria Rate of dissolving = rate of undissolving 3) Chemical equilibria (needs a chemical reaction) Rf = Rf (dynamic equilibria = always moving) Example: 2NO4 N2O4 this equilibria is chemical/dynamic equilibria Rules of writing the expression constant expression 1) Balance 2) Product over reactant 3) Use square brackets 4) Write Coefficients as powers 5) Make equation equal to Keq 1 - 5 relate to the law of mass action Note pure solids and liquids are never written in the equation so for N2(g) + 3H2(g) 2NH3(g) Keq = [NH3]^2 / [N2][H2]^3 Homogenous equilibrium: all in the same phase Heterogeneous equilibrium: different phases Application of Keq: 1. It predicts the direction of a reaction K >> 1 Products are favored K << 1 Reactants are favored K = 1 Equilibrium 2. You can find the equilibrium concentrations. 3. You can find the known concentrations The equilibrium expression for rxn is the reciprocal of that for the rxn written in reverse 2. Knew = (Koriginal)^n n = factor multiplied to original Ex: H2 + I2 = 2HI Keq = 50 1. HI -> H2 + H2 Keq = 1/50 2. 2(H2 + I2 --> HI) Keq = 50^2 Keq: It is the ration of the product concentration to the reactant concentration with each concentration raised to a power given by the number of moles of that substance in the balanced equation Reaction Quotient (Q): Q is obtained by applying the law of mass action using the initial concentration If K > Q more products If K < Q more reactants If K = Q at equilibrium Le Chatlelier's Principle: is a system is subjected to stress it will move in a direction to undo the stress 1) Temperature: - add heat to the system --> favors endothermic - lowering temperature in a system --> favors exothermic 2) Concentration: - more reactants --> move to the right --> products made - more products --> moves to the left --> reactants made 3) Pressure (only for gas) -increase pressure favors the rxn in a direction where volume is decreased (less moles of gas) For rxn's with equal moles of both sides, pressure and volume has no effect 4) Volume If Volume is decreased it goes to the direction with fewer moles If volume is increased it goes to the direction with high number of moles 5. Catalyst -increases the rate of the forward and reverse reaction (equilibrium will just be achieved faster) ------> Adding an inert gas (noble gas) has no effect (because it does not react) Only increases partial pressure Example: PCl3 + Cl3 + heat PCl5 - adding Cl2 --> -adding PCl5 <-- removing PCl5 --> -adding PCL2 --> -increasing temperature --> - lowering pressure <--adding catalyst - increasing pressure --> -lowering pressure <--removing PCl4 <--adding inert gas (no effect) - reduce volume --> Complete ionic equation: chemical equations that shows all soluble ionic substances as ions Net ionic equation: chemical equation that shows only those compounds and ions that undergo a chemical change in an aqueous solution Spectator ions: ions that do not take part in the reaction (Complete ion equation) - (spectator ions) = net ionic equation