A scientist named Henry Mosley developed the modern period table
... A scientist named Henry Mosley developed the modern period table. Mosley discovered if the elements were arranged a certain way, predictions could be made about the structure and properties of elements. Today we will investigate these patterns and discover that the periodic table is a useful tool wh ...
... A scientist named Henry Mosley developed the modern period table. Mosley discovered if the elements were arranged a certain way, predictions could be made about the structure and properties of elements. Today we will investigate these patterns and discover that the periodic table is a useful tool wh ...
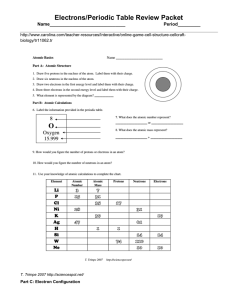
Electrons/Periodic Table Review Packet Name______________________________ Period_________
... b. metals with unpredictable properties c. a halogen d. make good semiconductors e. alkali metals f. ...
... b. metals with unpredictable properties c. a halogen d. make good semiconductors e. alkali metals f. ...
The Periodic Table - Anderson High School
... number of valence electrons. • They will form the same kinds of ions. ...
... number of valence electrons. • They will form the same kinds of ions. ...
The Periodic Table
... Each element has a different atomic mass. This number increases as you go across the rows from top to bottom of the table. ...
... Each element has a different atomic mass. This number increases as you go across the rows from top to bottom of the table. ...
Periodic Table Ch4 Honors
... History of the Periodic Table • In 1869, Dmitri Mendeleev, a Russian chemist, used Newland’s observations and other observations to produce the first PERIODIC Table. • He placed each element on a card with the element’s atomic mass, chemical and physical properties. He arranged the elements in many ...
... History of the Periodic Table • In 1869, Dmitri Mendeleev, a Russian chemist, used Newland’s observations and other observations to produce the first PERIODIC Table. • He placed each element on a card with the element’s atomic mass, chemical and physical properties. He arranged the elements in many ...
Science 2nd prep. 1st term Lesson 2 Graduation of the properties of
... reaction. To reach the nearest inert gas next them in the periodic table, ...
... reaction. To reach the nearest inert gas next them in the periodic table, ...
Document
... properties of elements that hadn’t yet been discovered. The Periodic Table was an important step in understanding elements’ properties. ...
... properties of elements that hadn’t yet been discovered. The Periodic Table was an important step in understanding elements’ properties. ...
Periodic Table
... 1. In the past people believed that there were only 4 elements now we know that there are over 100 elements 2. Everything is made of elements 3. A reason of your own. ...
... 1. In the past people believed that there were only 4 elements now we know that there are over 100 elements 2. Everything is made of elements 3. A reason of your own. ...
Rem001 - The Vital Chemist
... instance, has oxidation numbers (state) of +3, +4, +5. Manganese exhibits +2, +4, +6, +7 oxidation states amongst others. The 3d and 4s orbitals have very close energy levels. Therefore d-block elements can place their electrons in either the 3d or 4s orbitals for bond formation. As the variation in ...
... instance, has oxidation numbers (state) of +3, +4, +5. Manganese exhibits +2, +4, +6, +7 oxidation states amongst others. The 3d and 4s orbitals have very close energy levels. Therefore d-block elements can place their electrons in either the 3d or 4s orbitals for bond formation. As the variation in ...
5.3 Representative Groups - Chemistry with Mr. Saval
... Group 5A contains two nonmetals (nitrogen and phosphorus), two metalloids (arsenic and antimony), and one metal (bismuth). Group 5A includes elements with a wide range of physical properties. Nitrogen is a nonmetal gas, phosphorus is a solid nonmetal, and bismuth is a dense metal. All of the element ...
... Group 5A contains two nonmetals (nitrogen and phosphorus), two metalloids (arsenic and antimony), and one metal (bismuth). Group 5A includes elements with a wide range of physical properties. Nitrogen is a nonmetal gas, phosphorus is a solid nonmetal, and bismuth is a dense metal. All of the element ...
Chapter 5- The Periodic Law
... 1. Hydrogen does not share the same properties as the elements in Group 1 2. Helium possesses special chemical stability because of its filled outer shell E. The d-Block Elements: Groups 3-12 ...
... 1. Hydrogen does not share the same properties as the elements in Group 1 2. Helium possesses special chemical stability because of its filled outer shell E. The d-Block Elements: Groups 3-12 ...
File
... except for Period 1. Across a period from left to right, the elements become ___________________ and ___________________ in their properties. o Most ____________________________________ are on the left side of the table (group 1) o Most ____________________________________ are on the right side (gro ...
... except for Period 1. Across a period from left to right, the elements become ___________________ and ___________________ in their properties. o Most ____________________________________ are on the left side of the table (group 1) o Most ____________________________________ are on the right side (gro ...
Chem.-Chapter-6-notes
... nearby dsublevel generally contain electrons Inner Transition Metals- an element in the lanthanide or actinide series; the highest occupied s sublevel and nearby f sublevel of its atoms generally contain electrons; also called inner transition element Classifying the Elements A. Squares in the Perio ...
... nearby dsublevel generally contain electrons Inner Transition Metals- an element in the lanthanide or actinide series; the highest occupied s sublevel and nearby f sublevel of its atoms generally contain electrons; also called inner transition element Classifying the Elements A. Squares in the Perio ...
Slide 1
... 2. The problem was not only how to isolate the elements but how to organize them to establish some type of pattern. 3. In 1829, J.W. Dobereiner published a classification system in which elements were grouped in triads. 4. A triad is any of several sets of three chemically similar elements, the atom ...
... 2. The problem was not only how to isolate the elements but how to organize them to establish some type of pattern. 3. In 1829, J.W. Dobereiner published a classification system in which elements were grouped in triads. 4. A triad is any of several sets of three chemically similar elements, the atom ...
Oct 17-Oct 21
... I can use the periodic table to identify the characteristics and properties of metals, nonmetals, and metalloids ...
... I can use the periodic table to identify the characteristics and properties of metals, nonmetals, and metalloids ...
Periodic Table Study Guide
... 9) What are the vertical columns on the periodic table called? (give both names) 10) What is special about elements in the same group? 11) What are the horizontal rows called? 12) What is the pattern of protons in the periodic table? 13) What is the pattern of atomic mass in the periodic table? 14) ...
... 9) What are the vertical columns on the periodic table called? (give both names) 10) What is special about elements in the same group? 11) What are the horizontal rows called? 12) What is the pattern of protons in the periodic table? 13) What is the pattern of atomic mass in the periodic table? 14) ...
Chapter 4 – Atomic Structure (Sec 4.2 pages 108
... Transition Metals • Some transition elements have more properties in common than elements in other groups. – Elements in the lanthanide and actinide series – These elements are so similar that chemists in the 1800s had difficulty separating them when they were found mixed together in nature. ...
... Transition Metals • Some transition elements have more properties in common than elements in other groups. – Elements in the lanthanide and actinide series – These elements are so similar that chemists in the 1800s had difficulty separating them when they were found mixed together in nature. ...
Chapter 5: Electrons
... Describe the periodic tables of Moseley and Mendeleev. Identify the various families of elements on the periodic table. State the trends in atomic radius, ionization energy, electron affinity and ion size with a group or period on the periodic table. Identify the relationship between these t ...
... Describe the periodic tables of Moseley and Mendeleev. Identify the various families of elements on the periodic table. State the trends in atomic radius, ionization energy, electron affinity and ion size with a group or period on the periodic table. Identify the relationship between these t ...
The Periodic Table
... element is called an ATOM. • An element is a PURE substance, containing only one kind of ATOM. • The PERIODIC TABLE is a list of all the elements that have been discovered and named, with each element listed in its own element square. • Elements are represented on the Periodic Table by a one or two ...
... element is called an ATOM. • An element is a PURE substance, containing only one kind of ATOM. • The PERIODIC TABLE is a list of all the elements that have been discovered and named, with each element listed in its own element square. • Elements are represented on the Periodic Table by a one or two ...
6.1 Development of the Modern Periodic Table Objectives: 1
... When an atom loses electrons, it becomes smaller. Electrons are shed from the outermost energy level making it smaller. Since the size of the negative charge decreases, the positively charged nucleus can pull harder on the electrons and get them closer to the nucleus. When an atom gains elec ...
... When an atom loses electrons, it becomes smaller. Electrons are shed from the outermost energy level making it smaller. Since the size of the negative charge decreases, the positively charged nucleus can pull harder on the electrons and get them closer to the nucleus. When an atom gains elec ...
Periodic Trends
... Anions are bigger than the atom they come from. Nonmetals form anions. Anions of ‘main’ groups elements have noble gas configuration. ...
... Anions are bigger than the atom they come from. Nonmetals form anions. Anions of ‘main’ groups elements have noble gas configuration. ...
Regents Chemistry NOTE PACKET
... http://www.scienceclarified.com/everyday/Real-Life-Chemistry-Vol-1/Alkaline-Earth-Metals-Real-life-applications.html ...
... http://www.scienceclarified.com/everyday/Real-Life-Chemistry-Vol-1/Alkaline-Earth-Metals-Real-life-applications.html ...
The Periodic Table
... • The ionization energy of an atom is the amount of energy required to remove an electron in the gaseous state. • In general, the ionization energy increases as you go from the bottom to the top in a group. • In general, the ionization energy increases as you go from left to right across a period of ...
... • The ionization energy of an atom is the amount of energy required to remove an electron in the gaseous state. • In general, the ionization energy increases as you go from the bottom to the top in a group. • In general, the ionization energy increases as you go from left to right across a period of ...
Periodic Table Notes.notebook
... of the chart have so many electrons that loosing or acquiring an electron is not as big a deal. This is due to the shielding affect where electrons in lower energy levels shield the positive charge of the nucleus from outer electrons resulting in those outer electrons not being as tightly bound ...
... of the chart have so many electrons that loosing or acquiring an electron is not as big a deal. This is due to the shielding affect where electrons in lower energy levels shield the positive charge of the nucleus from outer electrons resulting in those outer electrons not being as tightly bound ...