alkali metal
... The present organization of the elements is a product of the first periodic table published by Dmitri Mendeleev in 1869. The amazing accuracy of his predictions has been very important to chemists in this century. However, the basis of his arrangement was the atomic masses of the elements. This appr ...
... The present organization of the elements is a product of the first periodic table published by Dmitri Mendeleev in 1869. The amazing accuracy of his predictions has been very important to chemists in this century. However, the basis of his arrangement was the atomic masses of the elements. This appr ...
ch05_sec2_as - LCMR School District
... – metal: an element that is shiny and that conducts heat and electricity well – nonmetal: an element that conducts heat and electricity poorly – semiconductor (or metalloid): an element or compound that conducts electric current better than an insulator does but not as well as a conductor does ...
... – metal: an element that is shiny and that conducts heat and electricity well – nonmetal: an element that conducts heat and electricity poorly – semiconductor (or metalloid): an element or compound that conducts electric current better than an insulator does but not as well as a conductor does ...
Section 2: Exploring the Periodic Table The Periodic Table Section 2
... – metal: an element that is shiny and that conducts heat and electricity well – nonmetal: an element that conducts heat and electricity poorly – semiconductor (or metalloid): an element or compound that conducts electric current better than an insulator does but not as well as a conductor does ...
... – metal: an element that is shiny and that conducts heat and electricity well – nonmetal: an element that conducts heat and electricity poorly – semiconductor (or metalloid): an element or compound that conducts electric current better than an insulator does but not as well as a conductor does ...
The Periodic Table
... Number of valence electrons each atom has When outer levels are full, atoms are stable When they are not full, they react: gain, lose, or share 1 or 2 electrons ...
... Number of valence electrons each atom has When outer levels are full, atoms are stable When they are not full, they react: gain, lose, or share 1 or 2 electrons ...
The Periodic Table - Crestwood Local Schools
... The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physical properties. ...
... The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physical properties. ...
ATOMIC STRUCTURE - IGCSE STUDY BANK
... Typical Properties of Metallic Elements Physical properties of metals ...
... Typical Properties of Metallic Elements Physical properties of metals ...
VIBRATIONS AND WAVES
... The first periodic table is mostly credited to (5) ___________________. In his table, the elements were arranged according to increasing (6) ___________________. One important result of this table was that the existence and properties of undiscovered (7) _________________ could be predicted. The ele ...
... The first periodic table is mostly credited to (5) ___________________. In his table, the elements were arranged according to increasing (6) ___________________. One important result of this table was that the existence and properties of undiscovered (7) _________________ could be predicted. The ele ...
Unit 3 Periodic Table Vocabulary
... Unit 3 Periodic Table Vocabulary Alkali Metals - Any of a group of soft, white, low-density, low-melting, highly reactive metallic elements, including lithium, sodium, potassium, rubidium, cesium, and francium. Sentence: You can find alkali metals in the periodic table. ...
... Unit 3 Periodic Table Vocabulary Alkali Metals - Any of a group of soft, white, low-density, low-melting, highly reactive metallic elements, including lithium, sodium, potassium, rubidium, cesium, and francium. Sentence: You can find alkali metals in the periodic table. ...
Elements and the Periodic Table
... Mendeleev’s Observations • One of Mendeleev’s first observations was that some elements have similar chemical and physical properties. • For example, Flourine and chlorine, are both gases that irritate your lungs if you breathe them. • Silver and copper are both shiny metals that gradually tarnish ...
... Mendeleev’s Observations • One of Mendeleev’s first observations was that some elements have similar chemical and physical properties. • For example, Flourine and chlorine, are both gases that irritate your lungs if you breathe them. • Silver and copper are both shiny metals that gradually tarnish ...
File
... 5) Sodium is an element located in a group name called alkali metal and is in which column of the Period Table? A: 1st B: 6th C: 18th D: 24th 6) What is the difference between elements aligned in the first column versus the last column? A: Elements in the 1st column are less reactive than elements i ...
... 5) Sodium is an element located in a group name called alkali metal and is in which column of the Period Table? A: 1st B: 6th C: 18th D: 24th 6) What is the difference between elements aligned in the first column versus the last column? A: Elements in the 1st column are less reactive than elements i ...
THE PERIODIC TABLE and PERIODIC LAW
... • "Metals are losers" • Losing electrons makes them form an ION ...
... • "Metals are losers" • Losing electrons makes them form an ION ...
Our modern Periodic Table
... a) Chemists in the 19th century wished to organize elements b) Attempts focused on grouping elements with similar properties c) In 1867, Dimitri Mendeleev found patterns in the elements and organized them into a table d) The resulting table had holes for elements not yet discovered ...
... a) Chemists in the 19th century wished to organize elements b) Attempts focused on grouping elements with similar properties c) In 1867, Dimitri Mendeleev found patterns in the elements and organized them into a table d) The resulting table had holes for elements not yet discovered ...
Uint one - pisscience
... Uint one Lesson (1) Attempts of Elements Classification * Many attempts are made by scientists for classification of elements: 1- To be easily studied 2- To find the relation between elements and their chemical and physical properties. Mendeleev's Periodic Table: *It is the first real periodic table ...
... Uint one Lesson (1) Attempts of Elements Classification * Many attempts are made by scientists for classification of elements: 1- To be easily studied 2- To find the relation between elements and their chemical and physical properties. Mendeleev's Periodic Table: *It is the first real periodic table ...
Coloring the Periodic Table - Families
... scientist born in Tobolsk, Siberia in 1834, is known as the father of the periodic table of the elements. The periodic table of the elements is an important tool used by students and chemists around the world to help them understand and simplify the often complex world of chemical reactions. ...
... scientist born in Tobolsk, Siberia in 1834, is known as the father of the periodic table of the elements. The periodic table of the elements is an important tool used by students and chemists around the world to help them understand and simplify the often complex world of chemical reactions. ...
Chapter 5 The Periodic Table Section 1 Organizing the Elements
... • The halogens combine easily with metals to form salts. • Nonmetals and their compounds are plentiful on Earth. ...
... • The halogens combine easily with metals to form salts. • Nonmetals and their compounds are plentiful on Earth. ...
Unit Six: Atomic structure
... Copper, Cu, is a relatively soft metal, and a very good electrical conductor. ...
... Copper, Cu, is a relatively soft metal, and a very good electrical conductor. ...
Chapter 5 The Periodic Law
... Name the block and group in which each of the following elements is located in the periodic table. Then, use the periodic table to name each element. Identify each element as a metal, nonmetal, or metalloid. Finally, describe whether each element has high reactivity or low reactivity. a. [Xe]4f145d9 ...
... Name the block and group in which each of the following elements is located in the periodic table. Then, use the periodic table to name each element. Identify each element as a metal, nonmetal, or metalloid. Finally, describe whether each element has high reactivity or low reactivity. a. [Xe]4f145d9 ...
2013 The Periodic Table
... The atomic radius of a chemical element is a measure of the size of its atoms, usually the mean or typical distance from the nucleus to the boundary of the surrounding cloud of electrons. ...
... The atomic radius of a chemical element is a measure of the size of its atoms, usually the mean or typical distance from the nucleus to the boundary of the surrounding cloud of electrons. ...
Periodic Table Metals, Non
... Notes on the Periodic Table of Elements The Periodic Table provides information on the physical and chemical properties of elements ...
... Notes on the Periodic Table of Elements The Periodic Table provides information on the physical and chemical properties of elements ...
C1a 1.1 Atoms, Elements and Compounds
... • Mendeleev arranged elements in order of atomic mass and took account of similar properties. He left gaps for undiscovered elements and even predicted the properties these missing elements should have. ...
... • Mendeleev arranged elements in order of atomic mass and took account of similar properties. He left gaps for undiscovered elements and even predicted the properties these missing elements should have. ...
The Periodic Table
... lowers activation energy so rxns are faster not used up in the rxn They are silvery-blue at room temperature (except copper and gold) - lustrous. Malleable They are solids at room temperature (except Hg) Good conductors of electricity (Why?) ...
... lowers activation energy so rxns are faster not used up in the rxn They are silvery-blue at room temperature (except copper and gold) - lustrous. Malleable They are solids at room temperature (except Hg) Good conductors of electricity (Why?) ...
Chapter 4
... The __________________ metals constitute Groups 3 through 12 and are sometimes called the d-block elements because of their position in the periodic table. Generally, the transition metals are less ___________ than the alkali metals and the alkaline-earth metals are. The elements in the first of th ...
... The __________________ metals constitute Groups 3 through 12 and are sometimes called the d-block elements because of their position in the periodic table. Generally, the transition metals are less ___________ than the alkali metals and the alkaline-earth metals are. The elements in the first of th ...
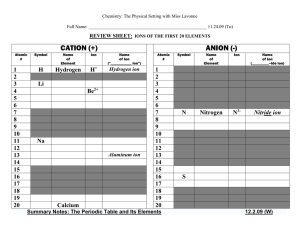
IONS OF THE FIRST 20 ELEMENTS
... The alkaline earth elements are metallic elements found in the second group of the periodic table. All alkaline earth elements have an oxidation number of +2, making them very reactive. Because of their reactivity, the alkaline metals are not found free in nature. The Alkaline Earth Metals are: Bery ...
... The alkaline earth elements are metallic elements found in the second group of the periodic table. All alkaline earth elements have an oxidation number of +2, making them very reactive. Because of their reactivity, the alkaline metals are not found free in nature. The Alkaline Earth Metals are: Bery ...
PERIODIC TABLE OF THE ELEMENTS
... · Have both metallic and non · Can be solid, liquid or gas metallic properties Have shiny appearance · Have dull appearance · semiconductors Good conductors of heat · Poor conductors of heat and and electricity electricity Are malleable and ductile · Are brittle (if solid) Lose electrons i ...
... · Have both metallic and non · Can be solid, liquid or gas metallic properties Have shiny appearance · Have dull appearance · semiconductors Good conductors of heat · Poor conductors of heat and and electricity electricity Are malleable and ductile · Are brittle (if solid) Lose electrons i ...