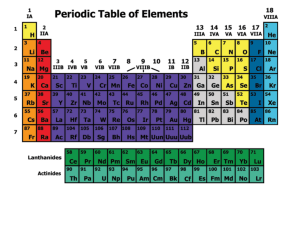
The Periodic Table of Elements
... called Alkali metals - the metals react with water to form alkaline solutions: the solutions turn red litmus paper blue have one outer shell electrons shiny, silvery solids soft, easily cut with scalpel low densities and melting points - these increases down the group good conductors of heat and ele ...
... called Alkali metals - the metals react with water to form alkaline solutions: the solutions turn red litmus paper blue have one outer shell electrons shiny, silvery solids soft, easily cut with scalpel low densities and melting points - these increases down the group good conductors of heat and ele ...
Homework Answers - Chemistry from AZ
... horizontal rows called periods are numbered 1 to 7; elements in the same period have the same number of principle energy levels (PEL’s) or shells vertical columns called groups or families, are numbered 1 to 18; elements in the same group have the same number of valence electrons and therefore have ...
... horizontal rows called periods are numbered 1 to 7; elements in the same period have the same number of principle energy levels (PEL’s) or shells vertical columns called groups or families, are numbered 1 to 18; elements in the same group have the same number of valence electrons and therefore have ...
The Periodic Table - Ms. Simmons
... • most clearly illustrate periodic patterns • most abundant naturally occurring elements • most important elements for living things, including most common components of ...
... • most clearly illustrate periodic patterns • most abundant naturally occurring elements • most important elements for living things, including most common components of ...
Periodicity PPt
... allows us to predict oxidation numbers for elements. Oxidation numbers represent the charge an ion obtains after losing or gaining valence electrons. ...
... allows us to predict oxidation numbers for elements. Oxidation numbers represent the charge an ion obtains after losing or gaining valence electrons. ...
Powerpoint for Periodicity and Density
... allows us to predict oxidation numbers for elements. Oxidation numbers represent the charge an ion obtains after losing or gaining valence electrons. ...
... allows us to predict oxidation numbers for elements. Oxidation numbers represent the charge an ion obtains after losing or gaining valence electrons. ...
Bohr Model Activity
... e. Use a different colour to highlight the electrons in the outermost shell. ...
... e. Use a different colour to highlight the electrons in the outermost shell. ...
PPT Test Review
... Answer the following questions. Number 1 – 34 and write your answers on a separate piece of paper. ...
... Answer the following questions. Number 1 – 34 and write your answers on a separate piece of paper. ...
Periodic Table Notes 1
... Groups ○ ________________ of the periodic table ○ Members of the groups have similar __________________ properties. _________________________: the pattern of ______________ properties displayed by elements in the periodic table. A unique ______________ for each element that equals the number of ____ ...
... Groups ○ ________________ of the periodic table ○ Members of the groups have similar __________________ properties. _________________________: the pattern of ______________ properties displayed by elements in the periodic table. A unique ______________ for each element that equals the number of ____ ...
Water Metal Hydroxide + Hydrogen
... The atomic radius of an atom is defined as the distance of closest approach to another atom and is the distance at which the mutual repulsion of the electron clouds and the mutual attraction of the nuclear charge of each for the electrons of the other are in equilibrium. The size of an atom in a mol ...
... The atomic radius of an atom is defined as the distance of closest approach to another atom and is the distance at which the mutual repulsion of the electron clouds and the mutual attraction of the nuclear charge of each for the electrons of the other are in equilibrium. The size of an atom in a mol ...
Chapter 6 Study Guide Key
... Ionization energy decreases down a group on the periodic table. The reason for this is shielding—as you move down a group the number of inner electrons increases as more energy levels are added. The greater number of inner electrons blocks the attractive forces between the nucleus and the valence el ...
... Ionization energy decreases down a group on the periodic table. The reason for this is shielding—as you move down a group the number of inner electrons increases as more energy levels are added. The greater number of inner electrons blocks the attractive forces between the nucleus and the valence el ...
Chapter 6 Notes
... current, similar to nonmetals But, when mixed with boron it is a good conductor of electric current, similar to metals ...
... current, similar to nonmetals But, when mixed with boron it is a good conductor of electric current, similar to metals ...
notes - unit 3 - periodic table_student_2014
... blue when dissolved in water) This concept is ________ on the __________________!!! Tend to be _________________ almost every transition metal can _________________________________ depending on what other elements or polyatomic ions are present Have multiple oxidation states _________ reac ...
... blue when dissolved in water) This concept is ________ on the __________________!!! Tend to be _________________ almost every transition metal can _________________________________ depending on what other elements or polyatomic ions are present Have multiple oxidation states _________ reac ...
Groups
... Elements that exist in more than one form are called allotropes. An example is carbon in forms of ...
... Elements that exist in more than one form are called allotropes. An example is carbon in forms of ...
Periodic Table
... 1860’s a Russian Scientist named Dmitri Mendeleev discovered a system for organizing all of the known elements. To help him find a pattern he put all of the known information on individual cards. He listed the elements known properties. ...
... 1860’s a Russian Scientist named Dmitri Mendeleev discovered a system for organizing all of the known elements. To help him find a pattern he put all of the known information on individual cards. He listed the elements known properties. ...
The Periodic Table
... Periods = Horizontal rows #’d 1-7 Each period contains more and more elements ...
... Periods = Horizontal rows #’d 1-7 Each period contains more and more elements ...
UNIT 4 NOTES: THE PERIODIC TABLE
... 2. They are __________ at reactive as other metals. 3. They form _______________ solutions when dissolved in water. For example Cu+2 is ___________, while Fe3+ is ___________. In other words transition elements have _______________ ions. 4. They have _____________________ positive oxidation states. ...
... 2. They are __________ at reactive as other metals. 3. They form _______________ solutions when dissolved in water. For example Cu+2 is ___________, while Fe3+ is ___________. In other words transition elements have _______________ ions. 4. They have _____________________ positive oxidation states. ...
4.3 Exploring the Modern Periodic Table
... a) How is the electron arrangement similar for these two elements? (Hint: look at the outer shell) b) The elements Br and I are also in the second last column of the periodic table. How many electrons do you think would be in their outer electron shell? 8. Elements in the same column tend to form si ...
... a) How is the electron arrangement similar for these two elements? (Hint: look at the outer shell) b) The elements Br and I are also in the second last column of the periodic table. How many electrons do you think would be in their outer electron shell? 8. Elements in the same column tend to form si ...
Essential Standard: 8.P.1 Understand the properties of matter and
... modern day table. Mendeleev was not the first to suggest a table, but he was the first to create one that predicted the existence of as-yet-undiscovered elements which were later discovered. As of 2012, the periodic table contains 118 confirmed chemical elements, of which 114 have been recognized by ...
... modern day table. Mendeleev was not the first to suggest a table, but he was the first to create one that predicted the existence of as-yet-undiscovered elements which were later discovered. As of 2012, the periodic table contains 118 confirmed chemical elements, of which 114 have been recognized by ...
Chapter 3: Atoms & the Periodic Table
... –iron, copper, nickel, silver, and gold • Most are hard and shiny • All are good conductors of electricity • Are fairly stable, reacting slowly or not at all with air and water ...
... –iron, copper, nickel, silver, and gold • Most are hard and shiny • All are good conductors of electricity • Are fairly stable, reacting slowly or not at all with air and water ...
Introduction To The Periodic Table Of The Elements
... React rapidly when exposed to air and water ...
... React rapidly when exposed to air and water ...
Slide 1
... The electrons that are furthest away can be shared or transferred to other atoms Valence electrons- electrons that are involved in transfer or sharing Elements have different numbers of valence electrons. These electrons will increase from left to right across a period ...
... The electrons that are furthest away can be shared or transferred to other atoms Valence electrons- electrons that are involved in transfer or sharing Elements have different numbers of valence electrons. These electrons will increase from left to right across a period ...
Ch 5
... Which of these elements are in the same period? Which are in the same group? Which elements would you expect to have the highest first ionization energy? Which would you expect to have the lowest? Which of the elements is most likely to for a 1+ ion? ...
... Which of these elements are in the same period? Which are in the same group? Which elements would you expect to have the highest first ionization energy? Which would you expect to have the lowest? Which of the elements is most likely to for a 1+ ion? ...
Notes: Unit 4: Periodic Table - Mr. Palermo`s Flipped Chemistry
... differ among elements. (3.1w) Elements can be differentiated by chemical properties. Chemical properties describe how an element behaves during a chemical reaction. (3.1x) Some elements exist in two or more forms in the same phase. These forms differ in their molecular or crystal structure, and henc ...
... differ among elements. (3.1w) Elements can be differentiated by chemical properties. Chemical properties describe how an element behaves during a chemical reaction. (3.1x) Some elements exist in two or more forms in the same phase. These forms differ in their molecular or crystal structure, and henc ...
Chapter 5: What you should know when you finish. Describe the
... Aluminum is strong, lightweight, malleable, and a good conductor of electric current Glass that contains boron is used to make laboratory glassware, such as the flasks. It is also used in cookware that can go directly from the oven to the refrigerator The Carbon Family – The elements of Group 4A ...
... Aluminum is strong, lightweight, malleable, and a good conductor of electric current Glass that contains boron is used to make laboratory glassware, such as the flasks. It is also used in cookware that can go directly from the oven to the refrigerator The Carbon Family – The elements of Group 4A ...
Unit 3 - The Periodic Table
... Found in the ___________ of the periodic table (the D block) Form ___________________ in solution (ex: Cu is bright blue when dissolved in water) This concept is ALWAYS on the REGENTS EXAM!!! Tend to be _____________________ will lose electrons or gain them depending on what other ________ ...
... Found in the ___________ of the periodic table (the D block) Form ___________________ in solution (ex: Cu is bright blue when dissolved in water) This concept is ALWAYS on the REGENTS EXAM!!! Tend to be _____________________ will lose electrons or gain them depending on what other ________ ...