* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Groups
Survey
Document related concepts
Transcript
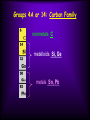
The Periodic Law Dmitri Mendeleev (1860) When the elements are placed in order by increasing atomic mass, their properties repeat in a periodic way. Moseley (~1910) Changed the elements’ order from atomic mass to atomic number. An atom’s chemical properties are determined by its valence (outside energy level) electrons. The identity of the element (what element an atom is) is determined by the number of protons it has. Protons Atoms tend to lose, gain or share electrons in order to get an electron configuration like a noble gas. Elements are arranged: Vertically into Groups Horizontally Into Periods Arrangement of Table Metals Metalloids Nonmetals Metals • Over 75% of the elements are classified as metals. • They are located to the left and lower parts of the periodic table. Properties of Metals a. Malleable a. Capable of being shaped or formed by being hammered b. c. d. e. Usually shiny, gray colored Conduct electricity Conduct heat Ductile a. Capable of being drawn out into a wire f. Usually solids at room temperature Metals Form Cations & Alloys A CATION forms when an atom loses one or more electrons. Mg --> Mg2+ + 2 e- Metals are mixed with other metals to form alloys. Stainless Steel Refrigerator Nonmetals • They are found in the upper right part of the table. Metals Metalloids Nonmetals Nonmetal properties are more diverse than the metals. • solid, liquid, or gas at room temp •usually poor heat conductors •usually poor electrical conductors •solids are brittle Nonmetals Form Anions An ANION forms when an atom gains one or more electrons Metalloids (also called semimetals) Metals Metalloids Nonmetals • They are found on the table between the metals and the nonmetals. •They have properties of both metals and nonmetals. Examples are silicon, arsenic, and germanium Periods (Energy Levels) Horizontally Rows • There are 7 periods in the periodic table. • The inner transition metals are actually “inside” periods 6 and 7. Periodic Trends • Across a period, atomic radius tends to decrease . • Across a period, ionization energy tends to increase . Elements are arranged: Vertically into Groups (also called chemical families) Groups • Eight tall groups of main group (representative) elements, numbers ending in A, or #1-2 & 13-18. • Ten shorter groups of transition metals, numbers ending in B or #3-12. • Elements in the same group have the same valence electron configuration, and therefore have similar chemical and physical properties. Down a group, atomic radius increases and ionization energy decreases. Increasing Increasing Group 1A: Alkali Metals Properties Group or Family Members Alkali Metals reacting with water: • • • • • Li (Lithium) – least reactive Na (Sodium) K (Potassium) Rb (Rubidium) Cs (Cesium) – more reactive What would you expect from Francium?!?! Group 1A: Alkali Metals Properties Valence electron configuration (period)s1 1) Soft, silvercolored metals 2) Highly reactive with H2O to form alkaline solution. Group 1A: Alkali Metals Properties cont. 3) They tend to form +1 ions Li --> Li1+ + 1 e- 4) Low density (some float in water They are part of the s block. Now get one color and outline the Alkali Metal Family. Group 2A: Alkaline Earth Metals Silvery-White Metals Many are found in rocks in the earth’s crust Group 2A: Alkaline Earth Metals cont. Valence electron configuration (period) s2 Group 2A: Alkaline Earth Metals Properties 1)react w/water to form alkaline solution 2)not as reactive as Group 1 3)not as soft as Group 1 4)tend to form +2 ions Mg --> Mg2+ + 2 e- Part of the s-block Now get one color and outline the Alkaline Earth Metal Family. Groups 3B through 2B or 3 through 12 Transition Metals Groups 3B through 2B or 3 through 12 Transition Metals Properties 1) often form colored compounds 2) harder than Groups 1 and 2 Groups 3B through 2B or 3 through 12 Transition Metals Many of these elements can form ions with varying charges, such as Fe+3/Fe+2, Cu+2/Cu+1 Part of the d-block Now get one color and outline the Transition Metals Groups 3A or 13: Boron Family (sometimes known as Aluminum Family) Valence electron configuration (period) s2 p1 Properties become more metallic down the group. Part of the p -block Now get one color and outline the Boron Family (sometimes known as Aluminum Family) Groups 4A or 14: Carbon Family nonmetals C metalloids Si, Ge metals Sn, Pb Groups 4A or 14: Carbon Family cont. Valence electron configuration (period) s2 p2 Properties become more metallic down the group. Part of the p -block Groups 4A or 14: Carbon Family cont. Elements that exist in more than one form are called allotropes. An example is carbon in forms of Graphite Diamonds Buckyballs Amorphic Now get one color and outline the Carbon Family Group 5A or 15: Nitrogen Family nonmetals N, P metalloids As, Sb metals Bi Group 5A or 15: Nitrogen Family Valence electron configuration (period) s2 p3 Properties become more metallic down the group. Nonmetals tend to form ions with - 3 charge. N --> N3- + 3 ePart of the p -block Now get one color and outline the Nitrogen Family Group 6A or 16: Oxygen Family nonmetals O, S, Se metalloid Te metals Po Valence electron configuration (period) s2 p4 Group 6A or 16: Oxygen Family Properties become more metallic down the group. Nonmetals tend to form ions with - 2 charge. O --> O2- + 2 ePart of the p -block Important allotropes from this group are oxygen in forms O2, oxygen gas and O3, ozone Now get one color and outline the Oxygen Family Group 7A or 17: Halogen Family (“saltmakers”) Members – all nonmetals F, Cl, Br, I, At Valence electron configuration (period) s2 p5 Part of the p -block Group 7A or 17: Halogen Family (“saltmakers”) cont. Very reactive, electronegative (measurement of the attraction for electrons) because these atoms need only one electron to have the same electron configuration as a noble gas. Tend to form ions with - 1 charge. F --> F1- + 1 e- Now get one color and outline the Halogen Family Group 8A or 18: Noble Gases Sometimes called “inert gases,” though most can be made to react. Valence electron configuration for He 1s2, all others (period) s2 p6 Group 8A or 18: Noble Gases cont. Properties 1. all are gases at room temp 2. relatively unreactive Part of the p -block They have no common ionic charge because they don’t form ions, have stable electron configurations Now get one color and outline the Noble Gases Compiled by Diddy Pedraza