Std 10th, Science and Technology, Maharashtra Board, English
... In addition to this, solved and practice problems are included which not only aim at covering the topic but also make students ready to face the competition. The topic-wise classified “question and answer” format of this book helps students in easy comprehension. Numerical problems included at the e ...
... In addition to this, solved and practice problems are included which not only aim at covering the topic but also make students ready to face the competition. The topic-wise classified “question and answer” format of this book helps students in easy comprehension. Numerical problems included at the e ...
Science - ExamResults.net
... In addition to this, solved and practice problems are included which not only aim at covering the topic but also make students ready to face the competition. The topic-wise classified “question and answer” format of this book helps students in easy comprehension. Numerical problems included at the e ...
... In addition to this, solved and practice problems are included which not only aim at covering the topic but also make students ready to face the competition. The topic-wise classified “question and answer” format of this book helps students in easy comprehension. Numerical problems included at the e ...
Document
... Humans and their ancestors have used stone tools made from brittle minerals for about 2.5 million years. The shattering of brittle minerals such as flint produced sharp edges useful for cutting and weaponry. Sometimes tools could be repaired by breaking a new cutting edge but the brittleness often m ...
... Humans and their ancestors have used stone tools made from brittle minerals for about 2.5 million years. The shattering of brittle minerals such as flint produced sharp edges useful for cutting and weaponry. Sometimes tools could be repaired by breaking a new cutting edge but the brittleness often m ...
School of Elements 1. - mt
... 3. The nuclear charge also increases but increase in number of shells dominates over increase in nuclear charge. So, atomic size increases down the group. Valency varies gradually across a period. 1. In the modern periodic table, the elements are arranged in increasing order of atomic number (Z). 2. ...
... 3. The nuclear charge also increases but increase in number of shells dominates over increase in nuclear charge. So, atomic size increases down the group. Valency varies gradually across a period. 1. In the modern periodic table, the elements are arranged in increasing order of atomic number (Z). 2. ...
A “periodic table” is an arrangement of elements in
... similar to one another and to other metals, but their properties do not fit in with those of any other family. Many transition metals combine chemically with oxygen to form compounds called oxides. ...
... similar to one another and to other metals, but their properties do not fit in with those of any other family. Many transition metals combine chemically with oxygen to form compounds called oxides. ...
Slide 1
... Recall that atoms form ions by either losing or gaining electrons. Like atomic size, ionic size has periodic trends. As you proceed down a group, the outermost electrons in ions are in higher energy levels. Therefore, just as atomic radius increases as you move down a group, usually the ionic radius ...
... Recall that atoms form ions by either losing or gaining electrons. Like atomic size, ionic size has periodic trends. As you proceed down a group, the outermost electrons in ions are in higher energy levels. Therefore, just as atomic radius increases as you move down a group, usually the ionic radius ...
C H A P T E R
... To understand why elements with similar properties appear at regular intervals in the periodic table, you need to examine the electron configurations of the elements. As shown in Figure 3, elements in each column of the table have the same number of electrons in their outer energy level. These elect ...
... To understand why elements with similar properties appear at regular intervals in the periodic table, you need to examine the electron configurations of the elements. As shown in Figure 3, elements in each column of the table have the same number of electrons in their outer energy level. These elect ...
CPO Science Link Teacher`s Guide
... neon (Ne), and argon (Ar). These elements do not naturally form chemical bonds with other atoms. They are almost always found in their pure state, not as part of compounds. Elements in Groups 3 through 12 are called the transition metals. These elements are usually good conductors of heat and electr ...
... neon (Ne), and argon (Ar). These elements do not naturally form chemical bonds with other atoms. They are almost always found in their pure state, not as part of compounds. Elements in Groups 3 through 12 are called the transition metals. These elements are usually good conductors of heat and electr ...
Valence Electrons - Warren County Public Schools
... Periodic Table: 4.30.14 Objectives: •Periodic Table Exam •I can predict chemical reactivity for an element based on its number of valence electrons and location on periodic table. • I can distinguish between metallic and non-metallic properties. •I can graph and interpret periodic trends. •I can un ...
... Periodic Table: 4.30.14 Objectives: •Periodic Table Exam •I can predict chemical reactivity for an element based on its number of valence electrons and location on periodic table. • I can distinguish between metallic and non-metallic properties. •I can graph and interpret periodic trends. •I can un ...
Introduction to Atoms
... 16. Is the following sentence true or false? The transition metals are less reactive than the metals in Groups 1 and 2. ________________________ 17. Is the following sentence true or false? All of the elements in Groups 13 through 15 are metals. ________________________ 18. Where are the lanthanides ...
... 16. Is the following sentence true or false? The transition metals are less reactive than the metals in Groups 1 and 2. ________________________ 17. Is the following sentence true or false? All of the elements in Groups 13 through 15 are metals. ________________________ 18. Where are the lanthanides ...
OXIDATION AND REDUCTION
... Oxidation Number is the valency of an atom in a molecule or ion which is assigned the sign either + or -. It may be (i)electrovalency or (ii)covalency . When the proper sign is associated with valency it becomes oxidation number(ON). It is mostly a theoretical concept particularly for covalent compo ...
... Oxidation Number is the valency of an atom in a molecule or ion which is assigned the sign either + or -. It may be (i)electrovalency or (ii)covalency . When the proper sign is associated with valency it becomes oxidation number(ON). It is mostly a theoretical concept particularly for covalent compo ...
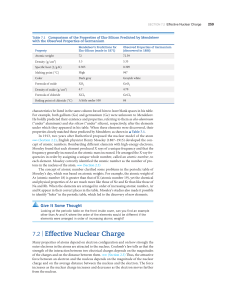
7.2 | Effective Nuclear Charge
... characteristics be listed in the same column forced him to leave blank spaces in his table. For example, both gallium (Ga) and germanium (Ge) were unknown to Mendeleev. He boldly predicted their existence and properties, referring to them as eka-aluminum (“under” aluminum) and eka-silicon (“under” s ...
... characteristics be listed in the same column forced him to leave blank spaces in his table. For example, both gallium (Ga) and germanium (Ge) were unknown to Mendeleev. He boldly predicted their existence and properties, referring to them as eka-aluminum (“under” aluminum) and eka-silicon (“under” s ...
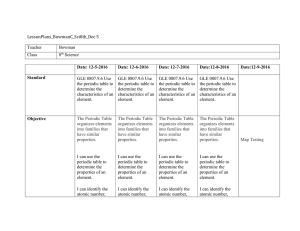
LessonPlans_BowmanC_Sci8th_Dec 5 Teacher Bowman Class 8th
... Group 6. Nitrogen Group 7. Oxygen Group 8. Halogens 9. Noble Gases 10. Hydrogen ...
... Group 6. Nitrogen Group 7. Oxygen Group 8. Halogens 9. Noble Gases 10. Hydrogen ...
Electrons in Atoms - Effingham County Schools
... An element has the electron configuration [Kr] 5s24d4. Without looking at the periodic table, identify the period, block, and group in which this element is located. Then, consult the periodic table to identify this element and the others in its group ...
... An element has the electron configuration [Kr] 5s24d4. Without looking at the periodic table, identify the period, block, and group in which this element is located. Then, consult the periodic table to identify this element and the others in its group ...
8th Grade Chap 4 Study Guide Answer Section
... 21. A material is said to be ductile if it a. is a mixture of a metal with at least one c. can be pulled out, or drawn, into a long other element. wire. b. can be hammered or rolled into flat sheets d. can transfer heat or electricity to another and other shapes. material. 22. The two most common al ...
... 21. A material is said to be ductile if it a. is a mixture of a metal with at least one c. can be pulled out, or drawn, into a long other element. wire. b. can be hammered or rolled into flat sheets d. can transfer heat or electricity to another and other shapes. material. 22. The two most common al ...
Periodic_table_questions
... Will the reaction be more reactive or less reactive than with lithium? ...
... Will the reaction be more reactive or less reactive than with lithium? ...
The Periodic Table and Chemical Properties
... elements having similar properties be grouped together? What sort of properties could be used? In 1867, a Russian chemist and teacher, Dmitri Mendeleev (Figure 2.10), wrote down the name of every known element on a separate card, like the one shown in Figure 2.11. He also wrote down properties he th ...
... elements having similar properties be grouped together? What sort of properties could be used? In 1867, a Russian chemist and teacher, Dmitri Mendeleev (Figure 2.10), wrote down the name of every known element on a separate card, like the one shown in Figure 2.11. He also wrote down properties he th ...
Chapter 23 Metals and Metallurgy
... • Transition metals often have more than one common oxidation state. Most have +2 state due to loss of s electrons. Oxidation numbers greater than 2 are due to loss of d electrons as well as s. Metals and Metallurgy ...
... • Transition metals often have more than one common oxidation state. Most have +2 state due to loss of s electrons. Oxidation numbers greater than 2 are due to loss of d electrons as well as s. Metals and Metallurgy ...
Periodicity of Elements and Periodic Table CHAPTER – 4
... Drawback or Defect As very few elements could be arranged in such groups, this classification did not get wide acceptance. Law of Octaves An English Chemist Newland (1864) stated that if the elements were arranged in the ascending order of their atomic masses, every eight element will have similar p ...
... Drawback or Defect As very few elements could be arranged in such groups, this classification did not get wide acceptance. Law of Octaves An English Chemist Newland (1864) stated that if the elements were arranged in the ascending order of their atomic masses, every eight element will have similar p ...
Periodic Trends Studyguide with Questions and Answers
... . Almost all metals are solids. Exception is mercury (Hg) , which is a liquid metal . Solid metals are malleable, ductile, and have luster . Metals tend to have high heat (thermal) and electrical conductivity due to their mobile valance electrons . Metals tend to have low electronegativity values (b ...
... . Almost all metals are solids. Exception is mercury (Hg) , which is a liquid metal . Solid metals are malleable, ductile, and have luster . Metals tend to have high heat (thermal) and electrical conductivity due to their mobile valance electrons . Metals tend to have low electronegativity values (b ...
Document
... • The elements were first organized by increasing atomic mass, which led to inconsistencies. Later, they were organized by increasing atomic number. • The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physica ...
... • The elements were first organized by increasing atomic mass, which led to inconsistencies. Later, they were organized by increasing atomic number. • The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physica ...
KHOA: HÓA HỌC - CCS - Trường Đại học Sư phạm Hà Nội
... element is a substance comprised of a single type of atom. The elements are the building blocks of our nature. An element is either discovered in nature or synthesized in the laboratory in pure form that cannot be separated into simpler substances by chemical methods. Currently, there are about 118 ...
... element is a substance comprised of a single type of atom. The elements are the building blocks of our nature. An element is either discovered in nature or synthesized in the laboratory in pure form that cannot be separated into simpler substances by chemical methods. Currently, there are about 118 ...
Study Guide for Electrons Mini-Test - seys
... - gold and silver are easily shaped and do not react easily = have been used for thousands of years to make jewelry and coins - ancient artifacts made of transition metals = unchanged since time made - among these metals are some of the earliest known elements - copper, gold, silver, iron - examples ...
... - gold and silver are easily shaped and do not react easily = have been used for thousands of years to make jewelry and coins - ancient artifacts made of transition metals = unchanged since time made - among these metals are some of the earliest known elements - copper, gold, silver, iron - examples ...