* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Slide 1
Survey
Document related concepts
Transcript
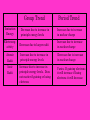
Chapter 4 The Periodic Table Chapter 4 – The Periodic Table Section 1 - How Are Elements Organized? Pure elements at room temperature and atmospheric pressure can be solids, liquids, or gases. Some elements are colorless. Others are colored. Despite the differences between elements, groups of elements share certain properties. Similarly, the elements fluorine, chlorine, bromine, and iodine can combine with sodium in a 1:1 ratio to form NaF, NaCl, NaBr, and NaI. These compounds are also white solids that can dissolve in water to form solutions that conduct electricity. These examples show that even though each element is different, groups of them have much in common. Chapter 4 – The Periodic Table Section 1 - How Are Elements Organized? In 1865, the English chemist John Newlands arranged the known elements according to their properties and in order of increasing atomic mass. He placed the elements in a table. In 1869, the Russian chemist Dmitri Mendeleev used Newlands’s observation and other information to produce the first orderly arrangement, or periodic table, of all 63 elements known at the time. Mendeleev arranged the elements in order of increasing atomic mass. Mendeleev started a new row each time he noticed that the chemical properties of the elements repeated. Chapter 4 – The Periodic Table Section 1 - How Are Elements Organized? He placed elements in the new row directly below elements of similar chemical properties in the preceding row. He arrived at the pattern shown below. Two interesting observations can be made about Mendeleev’s table. First, Mendeleev’s table contains gaps that elements with particular properties should fill. He predicted the properties of the missing elements. Chapter 4 – The Periodic Table Section 1 - How Are Elements Organized? Second, the elements do not always fit neatly in order of atomic mass. For example, Mendeleev had to switch the order of tellurium, Te, and iodine, I, to keep similar elements in the same column. At first, he thought that their atomic masses were wrong. However, careful research by others showed that they were correct. Mendeleev could not explain why his order was not always the same. About 40 years after Mendeleev published his periodic table, an English chemist named Henry Moseley found a different physical basis for the arrangement of elements. Moseley realized that the periodic table should be arranged by atomic number, not atomic mass. When the elements were arranged by increasing atomic number, the discrepancies in Mendeleev’s table disappeared. Chapter 4 – The Periodic Table Section 1 - How Are Elements Organized? The periodic law states that the repeating physical and chemical properties of elements change periodically with their atomic number To understand why elements with similar properties appear at regular intervals in the periodic table, you need to examine the electron configurations of the elements. Elements in each column of the table have the same number of electrons in their outer energy level. These electrons are called valence electrons. A vertical column on the periodic table is called a group or family. A horizontal row on the periodic table is called a period. Chapter 4 – The Periodic Table Section 2 - Tour of the Periodic Table Elements in groups 1, 2, and 13–18 are known as the main group elements. Four groups within the main-group elements have special names. These groups are the alkali metals (Group 1), the alkaline-earth metals (Group 2), the halogens (Group 17), and the noble gases (Group 18). Chapter 4 – The Periodic Table Section 2 - Tour of the Periodic Table The Alkali Metals Make Up Group 1 Alkali metals are so named because they are metals that react with water to make alkaline solutions. All have a single valence electron Alkali metals are usually stored in oil to keep them from reacting with the oxygen and water in the air. Because of their high reactivity, Alkali metals are never found in nature as pure elements but are found combined with other elements as compounds. Chapter 4 – The Periodic Table Section 2 - Tour of the Periodic Table The Alkaline-Earth Metals Make Up Group 2 Like the alkali metals, the alkaline-earth metals are highly reactive, so they are usually found as compounds rather than as pure elements All have 2 valence electrons The alkaline-earth metals are not as reactive as alkali metals, however they are harder and have higher melting points. Chapter 4 – The Periodic Table Section 2 - Tour of the Periodic Table The Halogens make up Group 17 Halogens have 7 valence electrons The halogens are the most reactive group of nonmetal elements The halogens have a wide range of physical properties. Fluorine and chlorine are gases at room temperature, but bromine, is a liquid, and iodine and astatine are solids. Chapter 4 – The Periodic Table Section 2 - Tour of the Periodic Table The Noble Gases make up Group 18 The noble gases were once called inert gases because they were thought to be completely unreactive. The low reactivity of noble gases leads to some special uses. Helium, a noble gas, is used to fill blimps because it has a low density and is not flammable. Hydrogen Is in a Class by Itself Hydrogen is the most common element in the universe. It is estimated that about three out of every four atoms in the universe are hydrogen. Because it consists of just one proton and one electron, hydrogen behaves unlike any other element. Chapter 4 – The Periodic Table Section 2 - Tour of the Periodic Table The Transition metals make up Groups 3 through 12 Unlike the main-group elements, the transition metals in each group do not have identical outer electron configurations. Generally, the transition metals are less reactive than the alkali metals and the alkaline-earth metals are. Lanthanides and Actinides Part of the last two periods of transition metals are placed toward the bottom of the periodic table to keep the table conveniently narrow, The elements in the first of these rows are called the Lanthanides because their atomic numbers follow the element lanthanum. Likewise, elements in the row below the lanthanides are called Actinides because they follow actinium. Chapter 4 – The Periodic Table Section 2 - Tour of the Periodic Table Summary of properties of selected groups Group 1 – All have 1 valence electron, and will easily lose this electron to form 1+ ions Group 2 – All have 2 valence electrons, and will easily lose both electrons to form 2+ ions Group 13 – All have 3 valence electrons, and will easily lose all of them to Form 3+ ions Group 16 – all have 6 valence electrons and will easily gain 2 electron to Form 2- ions Group 17 – all have 7 valence electrons and will easily gain 1 electron to Form 1- ions Group 18 – have a full outer shell with 8 electrons and will not react with other atom easily. Chapter 4 – The Periodic Table Section 2 - Tour of the Periodic Table Transition elements – are able to have multiple ion charges. All Possible charges are positive due to lose of electrons. Many will form a colored aqueous solution. Chapter 4 – The Periodic Table Section 2 - Tour of the Periodic Table Most elements of the periodic table are called Metals All metals are excellent conductors of electricity. Metals are excellent conductors of heat. Metals are ductile and malleable. Ductile means that the metal can be squeezed out into a wire. Malleable means that the metal can be hammered or rolled into sheets. Metals tend to lose electrons to complete the octet rule. All metals are solid except mercury, which is a liquid. Chapter 4 – The Periodic Table Section 2 - Tour of the Periodic Table Most elements of the periodic table near groups 14 -18 are called Nonmetals All nonmetals are poor conductors of electricity. Metals are poor conductors of heat. Solid nonmetals are brittle and break easily Metals tend to gain electrons to complete the octet rule. Nonmetals are found in all three states of matter with bromine being the only liquid. Metalloids are elements that share properties of both metals and nonmetals Metalloids are elements B, Si, As, Te, At, Ge, Sb and Po Chapter 4 – The Periodic Table Section 2 - Tour of the Periodic Table Review Questions At STP which element has a definite shape and volume? 1. Ag 2. Hg 3. Ne 4. Xe Which element is a member of the halogen family? 1. K 2. B 3. I 4. S Chapter 4 – The Periodic Table Section 2 - Tour of the Periodic Table Review Questions The metalloids that are included in Group 15 are antimony (Sb) and 1. N 2. P 3. As 4. Bi A characteristic of most nonmetallic solids is that they are 1. brittle 2. ductile 3. malleable 4. conductors of electricity More than two-thirds of the elements of the Periodic Table are classified as 1. metalloids 2. metals 3. nonmetals 4. noble gases Chapter 4 – The Periodic Table Section 2 - Tour of the Periodic Table Review Questions To which group do the alkaline earth metals belong? 1. 1 2. 2 3. 11 4. 12 Which three elements have the most similar chemical properties? 1. Ar, Kr, Br 2. K, Rb, Cs 3. B, C, N 4. O, N, Si When metals form ions, they tend to do so by 1. losing electrons and forming positive ions 2. losing electrons and forming negative ions 3. gaining electrons and forming positive ions 4. gaining electrons and forming negative ions Chapter 4 – The Periodic Table Section 2 - Tour of the Periodic Table Review Questions The chemical properties of the elements are periodic functions of their atomic 1. masses 2. weights 3. numbers 4. radii Which halogen is a solid at STP? 1. fluorine 2. chlorine 3. bromine 4. iodine Which element is in Group 2 and Period 7 of the Periodic Table? 1. magnesium 2. manganese 3. radium 4. radon Chapter 4 – The Periodic Table Section 2 - Tour of the Periodic Table Review Questions An atom in the ground state contains 8 valence electrons. This atom is classified as a 1. metal 2. semimetal 3. noble gas 4. halogen Which category is composed of elements that have both positive and negative charges? 1. the alkali metals 2. the transition metals 3. the halogens 4. the alkaline earths Chapter 4 – The Periodic Table Section 2 - Tour of the Periodic Table Review Questions Which element is so active chemically that it occurs naturally only in compounds? 1. potassium 2. silver 3. copper 4. sulfur The elements of the Periodic Table are arranged in horizontal rows according to each successive element's greater 1. atomic mass 2. atomic radius 3. number of protons 4. number of neutrons Chapter 4 – The Periodic Table Section 2 - Tour of the Periodic Table Review Questions In which list are the elements arranged in order of increasing atomic mass? 1. Cl, K, Ar 2. Fe, Co, Ni 3. Te, I, Xe 4. Ne, F, Na Which Group of the Periodic Table contains atoms with a stable outer electron configuration? 1. 1 2. 8 3. 16 4. 18 The element in Period 4 and Group 1 of the Periodic Table would be classified as a 1. metal 2. metalloid 3. nonmetal 4. noble gas Chapter 4 – The Periodic Table Section 3 - Trends in the Periodic Table The arrangement of the periodic table reveals trends in the properties of the elements. A trend is a predictable change in a particular direction. Metallic Trend All of the elements of the periodic table increase in metallic characteristics as they get closer to francium. This will make group 1 the most metallic group and period 7 the most metallic period. Nonmetallic Trend All of the elements of the periodic table increase in nonmetallic characteristics as they get closer to flourine. This will make group 17 the most nonmetallic group and period 2 the most nonmetallic period. Note: The noble gases are not considered part of the metallic or nonmetallic trends due to there resistance to chemical reactions. Chapter 4 – The Periodic Table Section 3 - Trends in the Periodic Table Ionization Energy The energy that is supplied to remove an electron is the ionization energy of the atom. Chapter 4 – The Periodic Table Section 3 - Trends in the Periodic Table Ionization Energy Decreases as You Move Down a Group Each element has more occupied energy levels than the one above it has. Therefore, the outermost electrons are farthest from the nucleus in elements near the bottom of a group. Similarly, as you move down a group, each successive element contains more electrons in the energy levels between the nucleus and the outermost electrons. These inner electrons shield the outermost electrons from the full attractive force of the nucleus. This electron shielding causes the outermost electrons to be held less tightly to the nucleus. Chapter 4 – The Periodic Table Section 3 - Trends in the Periodic Table Ionization Energy Increases as You Move Across a Period From one element to the next in a period, the number of protons and the number of electrons increase by one each. A higher nuclear charge more strongly attracts the outer electrons in the same energy level, but the electron-shielding effect from inner-level electrons remains the same. Thus, more energy is required to remove an electron because the attractive force on them is higher. Chapter 4 – The Periodic Table Section 3 - Trends in the Periodic Table Chapter 4 – The Periodic Table Section 3 - Trends in the Periodic Table Atomic Radius The exact size of an atom is hard to determine. An atom’s size depends on the volume occupied by the electrons around the nucleus, and the electrons do not move in well-defined paths. Rather, the volume the electrons occupy is thought of as an electron cloud, with no clear-cut edge. In addition, the physical and chemical state of an atom can change the size of an electron cloud. The diagram below shows one way to measure the size of an atom. This Method involves calculating the bond radius, the length that is half the distance between the nuclei of two bonded atoms. The bond radius can change slightly depending on what atoms are involved. Chapter 4 – The Periodic Table Section 3 - Trends in the Periodic Table Atomic Radius Increases as You Move Down a Group As you proceed from one element down to the next in a group, another principal energy level is filled. The addition of another level of electrons increases the size, or atomic radius, of an atom. Atomic Radius Decreases as You Move Across a Period As you move from left to right across a period, each atom has one more proton and one more electron than the atom before it has. As a result, electron shielding does not play a role as you move across a period. Therefore, as the nuclear charge increases across a period, the effective nuclear charge acting on the outer electrons also increases. This increasing nuclear charge pulls the outermost electrons closer and closer to the nucleus and thus reduces the size of the atom. Chapter 4 – The Periodic Table Section 3 - Trends in the Periodic Table Chapter 4 – The Periodic Table Section 3 - Trends in the Periodic Table Electronegativity Atoms often bond to one another to form a compound. These bonds can involve the sharing of valence electrons. Not all atoms in a compound share electrons equally. Knowing how strongly each atom attracts bonding electrons can help explain the physical and chemical properties of the compound. Linus Pauling, one of America’s most famous chemists, made a scale of numerical values that reflect how much an atom in a molecule attracts electrons, called electronegativity values. Chemical bonding that comes from a sharing of electrons can be thought of as a tug of war. The atom with the higher electronegativity will pull on the electrons more strongly than the other atom will. Fluorine is the element whose atoms most strongly attract shared electrons in a compound. Pauling arbitrarily gave fluorine an electronegativity value of 4.0.Values for the other elements were calculated in relation to this value. Chapter 4 – The Periodic Table Section 3 - Trends in the Periodic Table Electronegativity Decreases as You Move Down a Group Metals tend to have small electronegativity because they would rather lose an electron than gain electrons to fulfill the octet rule. As you go down a group the distance between the valence electrons and the nucleus increase, weakening the “grip” the nucleus has on it valence electrons due to electron shielding. Electronegativity Increases as You Move Across a Period Nonmetals tend to have large electronegativity because they would rather gain an electron than lose electrons to fulfill the octet rule. As you go across a period the distance between the valence electrons and the nucleus decreases, strengthening the “grip” the nucleus has on it valence electrons. Chapter 4 – The Periodic Table Section 3 - Trends in the Periodic Table Chapter 4 – The Periodic Table Section 3 - Trends in the Periodic Table Periodic Trends in Ionic Size Recall that atoms form ions by either losing or gaining electrons. Like atomic size, ionic size has periodic trends. As you proceed down a group, the outermost electrons in ions are in higher energy levels. Therefore, just as atomic radius increases as you move down a group, usually the ionic radius increases as well. These trends hold for both positive and negative ions. Metals tend to lose one or more electrons and form a positive ion. As you move across a period, the ionic radii of metal cations tend to decrease because of the increasing nuclear charge. As you come to the nonmetal elements in a period, their atoms tend to gain electrons and form negative ions. The diagram on the next slide shows that as you proceed through the anions on the right of a period, ionic radii still tend to decrease because of the anions’ increasing nuclear charge. Chapter 4 – The Periodic Table Section 3 - Trends in the Periodic Table Summary of Ionic Radii Atoms that form anions will increase in ionic radius, due to the nuclear charge becoming less than the total electron cloud charge. Atoms that form cations will decrease in ionic radius due to the nuclear charge becoming greater than the electron cloud charge. All atoms forming anions or cations will increase ionic radius when moving down a group because of the addition of a principle energy level. Chapter 4 – The Periodic Table Section 3 - Trends in the Periodic Table Other information about the periodic table. All elements with an atomic number greater than 83 have isotopes that are not stable and will undergo nuclear decay. (They are radioactive) Chemistry Reference tables lists, electronegativity and ionizations energies and atomic radii. Chapter 4 – The Periodic Table Section 3 - Trends in the Periodic Table Which element forms an ion that is larger than its atom? 1. Cl 2. Ca 3. Li 4. Mg Which atom has a radius larger than the radius of its ion? 1. Cl 2. Ca 3. S 4. Se Which of the following elements in Period 3 has the greatest metallic character? 1. Ar 2. Si 3. Mg 4. S Chapter 4 – The Periodic Table Section 3 - Trends in the Periodic Table Which period of the Periodic Table contains more metallic elements than nonmetallic elements? 1. Period 1 2. Period 2 3. Period 3 4. Period 4 As the elements in Period 3 are considered from left to right, they tend to 1. lose electrons more readily and increase in metallic character 2. lose electrons more readily and increase in nonmetallic character 3. gain electrons more readily and increase in metallic character 4. gain electrons more readily and increase in nonmetallic character Compared to the nonmetals in Period 2, the metals in Period 2 generally have larger 1. ionization energies 2. electronegativities 3. atomic radii 4. atomic numbers Chapter 4 – The Periodic Table Section 3 - Trends in the Periodic Table Which of the following Group 2 elements has the lowest first ionization energy? 1. Be 2. Mg 3. Ca 4. Ba Which of the following atoms has the greatest tendency to attract electrons? 1. barium 2. beryllium 3. boron 4. bromine In which shell are the valence electrons of the elements in Period 2 found? 1. 1 2. 2 3. 3 4. 4 Chapter 4 – The Periodic Table Section 3 - Trends in the Periodic Table Which of the following ions has the smallest radius? 1. F2. Cl3. K+ 4. Ca2+ The ability of carbon to attract electrons is 1. greater than that of nitrogen, but less than that of oxygen 2. less than that of nitrogen, but greater than that of oxygen 3. greater than that of nitrogen and oxygen 4. less than that of nitrogen and oxygen Which trends appear as the elements in Period 3 are considered from left to right? 1. Metallic character decreases, and electronegativity decreases. 2. Metallic character decreases, and electronegativity increases. 3. Metallic character increases, and electronegativity decreases. 4. Metallic character increases, and electronegativity increases. Chapter 4 – The Periodic Table Section 3 - Trends in the Periodic Table As the atoms in Period 3 of the Periodic Table are considered from left to right, the atoms generally show 1. an increase in radius and an increase in ionization energy 2. an increase in radius and a decrease in ionization energy 3. a decrease in radius and an increase in ionization energy 4. a decrease in radius and a decrease in ionization energy Which Group 16 element has only unstable isotopes? 1. Po 2. Te 3. Se 4. S How much energy is required to remove the most loosely bound electron from a neutral atom of carbon in the gaseous phase? 1. 801 kJ/mol 2. 1086 kJ/mol 3. 1251 kJ/mol 4. 1000 kJ/mol Chapter 4 – The Periodic Table Section 3 - Trends in the Periodic Table THE END Review How is periodic table arranged: In order of atomic number not mass as the original periodic tables were. Define: Groups Go up and down on periodic table Periods Go across the periodic table Alkali Metals Group 1 Alkaline Earth Metals Group 2 Halogens Group 17 Noble Gases Group 18 Transition Elements Groups 3-12 Metals Nonmetals Location Left Right Conduct Electricity Yes No Conduct Heat Yes No Malleable Yes No Brittle (if solid) No Yes Tend to ____ Electrons Lose Gain Name the liquid Mercury Bromine Best represents Francium Fluorine Ionization Energy Electronegativity Atomic Radii Ionic Radii Energy required to remove one valence electron Willingness to gain an electron Size of an atom Size of an atom after it has gained/lost an electron(s) Group Trend Ionization Energy Decrease due to increase in principle energy levels Period Trend Increase due to increase in nuclear charge Electronegativity Decrease due to larger radii Increase due to increase in nuclear charge Atomic Radii Increase due to increase in principle energy levels Decrease due to increase in nuclear charge Ionic Radii Increase due to increase in principle energy levels. Does not matter if gaining or losing electrons Varies- If gaining electrons it will increase if losing electrons it will decrease