Name
... b. Noble Gases d. Alkali Metals _____ 5. The more distinctive property of the noble gases is that they are a. metallic c. metalloids b. reactive d. largely unreactive _____ 6. Lithium, the first element in Group 1, has an atomic number of 3. The second element in this group has an atomic number a. 4 ...
... b. Noble Gases d. Alkali Metals _____ 5. The more distinctive property of the noble gases is that they are a. metallic c. metalloids b. reactive d. largely unreactive _____ 6. Lithium, the first element in Group 1, has an atomic number of 3. The second element in this group has an atomic number a. 4 ...
TOC 9/18 Organization of Periodic Table
... Reactivity is a chemical property that determines how elements will react with others to form compounds. ...
... Reactivity is a chemical property that determines how elements will react with others to form compounds. ...
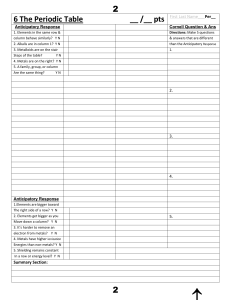
6 The Periodic Tableааааааааааааааааааааааааа__ /__ pts First
... Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. ________ 10. In his periodic table, Mendeleev arranged the elements in order of atomic number. ________ 11. There are six periods in a periodic table. ________ 12. Most of the elements in the periodic table ...
... Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. ________ 10. In his periodic table, Mendeleev arranged the elements in order of atomic number. ________ 11. There are six periods in a periodic table. ________ 12. Most of the elements in the periodic table ...
The Periodic Table
... less reactive, but similar to alkali metals; low electron affinity, CALCIUM – 5th most abundant element on earth (lime, calcium chloride, body functions) ...
... less reactive, but similar to alkali metals; low electron affinity, CALCIUM – 5th most abundant element on earth (lime, calcium chloride, body functions) ...
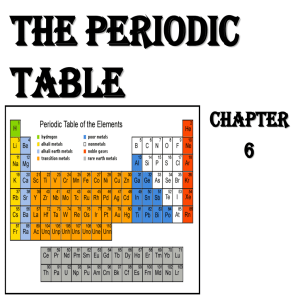
The periodic table shows all the elements and their
... The element symbol is always almost accompanied by other information such as atomic number and atomic weight. Atomic number describes the number of protons in one atomof that element. For example, an atom of oxygen contains 8 protons. Elements are listed in order of increasing atomic number from lef ...
... The element symbol is always almost accompanied by other information such as atomic number and atomic weight. Atomic number describes the number of protons in one atomof that element. For example, an atom of oxygen contains 8 protons. Elements are listed in order of increasing atomic number from lef ...
Intro to Periodic Table and Lewis Structures
... • The Periodic Law simply states that “when the elements are arranged by increasing ATOMIC NUMBER, their physical and chemical properties repeat at regular intervals”. • This is where the name PERIODIC TABLE comes from! ...
... • The Periodic Law simply states that “when the elements are arranged by increasing ATOMIC NUMBER, their physical and chemical properties repeat at regular intervals”. • This is where the name PERIODIC TABLE comes from! ...
Periodic Table - Mrs. Sousa`s Science Site
... Very Unreactive because they have full electron levels ...
... Very Unreactive because they have full electron levels ...
Power point notes - Social Circle City Schools
... metals and non-metals. They are solids that can be shiny or dull. They conduct heat and electricity better than nonmetals but not as well as metals. They are ductile and ...
... metals and non-metals. They are solids that can be shiny or dull. They conduct heat and electricity better than nonmetals but not as well as metals. They are ductile and ...
Groups in a Periodic Table
... The Representative Elements • These elements display a wide range of physical and chemical properties. • The atoms of the representative elements have “s” and “p” orbitals of the highest occupied energy levels that are not full. • For any element of this group, the group number (the American and Eu ...
... The Representative Elements • These elements display a wide range of physical and chemical properties. • The atoms of the representative elements have “s” and “p” orbitals of the highest occupied energy levels that are not full. • For any element of this group, the group number (the American and Eu ...
Unit 4 (2016 – 2017)
... 2. Transition Metals, Lanthanoids, and Actinoids for the elements in the family – list the range of atomic numbers --- do not name them all!! Example: Transition metals range from 21 – 30 and …. 3. Go to 1 other website – search for your family in a search engine to find information. For “other” inf ...
... 2. Transition Metals, Lanthanoids, and Actinoids for the elements in the family – list the range of atomic numbers --- do not name them all!! Example: Transition metals range from 21 – 30 and …. 3. Go to 1 other website – search for your family in a search engine to find information. For “other” inf ...
File
... the alkali metals in the same period! • So Mg is less reactive than Na, but more reactive than Be!!! • Location allows prediction of reactivity! ...
... the alkali metals in the same period! • So Mg is less reactive than Na, but more reactive than Be!!! • Location allows prediction of reactivity! ...
Alkaline Earth Metals
... How are sublevels and PELs related? How are orbitals and orbits related? What’s the formula for the maximum number of electrons allowed in a certain PEL? List the elements that exist as diatomics. What are the trends in atomic radius as you go down and across the periodic table? Why? What are the tr ...
... How are sublevels and PELs related? How are orbitals and orbits related? What’s the formula for the maximum number of electrons allowed in a certain PEL? List the elements that exist as diatomics. What are the trends in atomic radius as you go down and across the periodic table? Why? What are the tr ...
Study Material - Tiwariacademy.net
... Metallic character increases as we go down a group as the effective nuclear charge is decreasing. Non metals are electronegative. They tend to form bonds by gaining electrons. ...
... Metallic character increases as we go down a group as the effective nuclear charge is decreasing. Non metals are electronegative. They tend to form bonds by gaining electrons. ...
Chapter 5 Chem classnotes
... Ionization energy (IE) is the energy required to remove an electron from a neutral atom of an element. As atomic number increases going down a group, more electrons lie between the nucleus and the electrons in the outer orbits. This shields the outer electrons from the nuclear forces of attraction. ...
... Ionization energy (IE) is the energy required to remove an electron from a neutral atom of an element. As atomic number increases going down a group, more electrons lie between the nucleus and the electrons in the outer orbits. This shields the outer electrons from the nuclear forces of attraction. ...
Section 6.1 Development of the Modern Periodic Table
... • Metals are elements that are generally shiny when smooth and clean, solid at room temperature, and good conductors of heat and electricity. • Alkali metals are all the elements in group 1 except hydrogen, and are very reactive. • Alkaline earth metals are in group 2, and are also highly reactive. ...
... • Metals are elements that are generally shiny when smooth and clean, solid at room temperature, and good conductors of heat and electricity. • Alkali metals are all the elements in group 1 except hydrogen, and are very reactive. • Alkaline earth metals are in group 2, and are also highly reactive. ...
Untitled
... • Elements are classified as metals, non-metals, and metalloids. • Metals are elements that are generally shiny when smooth and clean, solid at room temperature, and good conductors of heat and electricity. • Alkali metals are all the elements in group 1 except hydrogen, and are very reactive. • Alk ...
... • Elements are classified as metals, non-metals, and metalloids. • Metals are elements that are generally shiny when smooth and clean, solid at room temperature, and good conductors of heat and electricity. • Alkali metals are all the elements in group 1 except hydrogen, and are very reactive. • Alk ...
MATTER AND PERIODIC TABLE
... positively charged protons and neutral neutrons. A cloud of negatively charged electrons surrounds the nucleus of an atom. ...
... positively charged protons and neutral neutrons. A cloud of negatively charged electrons surrounds the nucleus of an atom. ...
WILF 1 - GCSE Chemistry Help
... If elements are arranged in increasing order of atomic number, the problems with elements like tellurium and iodine are solved. Elements in the same group have the same number of outer shell electrons. It is the number of outer shell electrons which determines how the element behaves. Chlorine ...
... If elements are arranged in increasing order of atomic number, the problems with elements like tellurium and iodine are solved. Elements in the same group have the same number of outer shell electrons. It is the number of outer shell electrons which determines how the element behaves. Chlorine ...
8th Science LF Sept 7-11
... Color the periodic table to distinguish location of metals, non-metals, and metalloids. Think Pair Share – Identify Periodic Table Trends Discover that Element Game from Discovery Education (print pgs. 2 and 10) Quick Write – See Critical Writing Prompt ...
... Color the periodic table to distinguish location of metals, non-metals, and metalloids. Think Pair Share – Identify Periodic Table Trends Discover that Element Game from Discovery Education (print pgs. 2 and 10) Quick Write – See Critical Writing Prompt ...
Section 2: Exploring the Periodic Table
... – metal: an element that is shiny and that conducts heat and electricity well – nonmetal: an element that conducts heat and electricity poorly – semiconductor (or metalloid): an element or compound that conducts electric current better than an insulator does but not as well as a conductor does ...
... – metal: an element that is shiny and that conducts heat and electricity well – nonmetal: an element that conducts heat and electricity poorly – semiconductor (or metalloid): an element or compound that conducts electric current better than an insulator does but not as well as a conductor does ...
Periodic Trends PDF - Warren County Schools
... • This group of metals is extremely reactive! This is due to the fact that they all have only 1 valence electron. • In their pure state, all members of this group are silvery in appearance and soft enough to be cut with a butter knife. • Because they are so reactive, they are not found in natur ...
... • This group of metals is extremely reactive! This is due to the fact that they all have only 1 valence electron. • In their pure state, all members of this group are silvery in appearance and soft enough to be cut with a butter knife. • Because they are so reactive, they are not found in natur ...
The Periodic Table - Warren County Public Schools
... • This group of metals is extremely reactive! This is due to the fact that they all have only 1 valence electron. • In their pure state, all members of this group are silvery in appearance and soft enough to be cut with a butter knife. • Because they are so reactive, they are not found in nature as ...
... • This group of metals is extremely reactive! This is due to the fact that they all have only 1 valence electron. • In their pure state, all members of this group are silvery in appearance and soft enough to be cut with a butter knife. • Because they are so reactive, they are not found in nature as ...
Periodic table of elements
... All atoms (except hydrogen) want to have 8 electrons in their very outermost energy level (This is called the rule of octet.) Atoms bond until this level is complete. Atoms with few valence electrons lose them during bonding. Atoms with 6, 7, or 8 valence electrons gain electrons during bonding. ...
... All atoms (except hydrogen) want to have 8 electrons in their very outermost energy level (This is called the rule of octet.) Atoms bond until this level is complete. Atoms with few valence electrons lose them during bonding. Atoms with 6, 7, or 8 valence electrons gain electrons during bonding. ...