atoms
... • Protons and neutrons have essentially the same mass. • The mass of an electron is so small when compared to that of a proton or a neutron. We ignore it. • Protons and neutrons are found in the nucleus; electrons travel around the nucleus. ...
... • Protons and neutrons have essentially the same mass. • The mass of an electron is so small when compared to that of a proton or a neutron. We ignore it. • Protons and neutrons are found in the nucleus; electrons travel around the nucleus. ...
Unit 3 – Atomic Theory
... (fission = splitting). In this reaction, certain specific elements have their nuclei broken down into smaller parts. This reaction releases a tremendous amount of energy, which can be used for an explosion (nuclear weaponry), or to power and electric generator (nuclear reactor). ...
... (fission = splitting). In this reaction, certain specific elements have their nuclei broken down into smaller parts. This reaction releases a tremendous amount of energy, which can be used for an explosion (nuclear weaponry), or to power and electric generator (nuclear reactor). ...
Welcome to Chemistry 1001
... integer ratio. Its properties are different to those of the component elements. (eg. water, alcohol) • A mixture has different elements or compounds mingled together. The properties of the components are maintained. (eg. beer, martini) ...
... integer ratio. Its properties are different to those of the component elements. (eg. water, alcohol) • A mixture has different elements or compounds mingled together. The properties of the components are maintained. (eg. beer, martini) ...
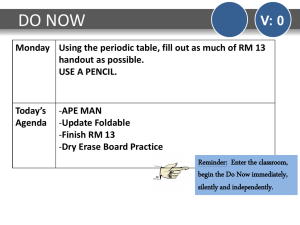
DO NOW - PBworks
... - What is one pattern going across? - What is one pattern going up and down? ...
... - What is one pattern going across? - What is one pattern going up and down? ...
ATOMIC STRUCTURE questions
... How many ELECTRONS are,there in each of the following ATOMS ? Sodium, Lithium, Ba,O, W, the element with an atomic number 2, the element with 87 protons in its nucleus. The MASS of an atom is contained in the NUCLEUS, the ATOMIC MASS or MASS NUMBER of an element is the SUM of the PROTONS and the NEU ...
... How many ELECTRONS are,there in each of the following ATOMS ? Sodium, Lithium, Ba,O, W, the element with an atomic number 2, the element with 87 protons in its nucleus. The MASS of an atom is contained in the NUCLEUS, the ATOMIC MASS or MASS NUMBER of an element is the SUM of the PROTONS and the NEU ...
Catalyst
... The mass number or atomic mass: This number tells the mass of one atom, which is approximately the sum of protons and neutrons in the nucleus, since each proton and each neutron has a mass equal to one mass unit, and the electrons ...
... The mass number or atomic mass: This number tells the mass of one atom, which is approximately the sum of protons and neutrons in the nucleus, since each proton and each neutron has a mass equal to one mass unit, and the electrons ...
1b Atomic Structure
... up with a full outer shell. Except for the first shell, which holds a maximum of two electrons, the second and third shells fill up with 8 electrons each. The tendency to fill up the outer shell with electrons is therefore often referred to as the Octet Rule: atoms will acquire or lose electrons in ...
... up with a full outer shell. Except for the first shell, which holds a maximum of two electrons, the second and third shells fill up with 8 electrons each. The tendency to fill up the outer shell with electrons is therefore often referred to as the Octet Rule: atoms will acquire or lose electrons in ...
Periodic Table Extra Practice ANSWER KEY 2014
... Periodic Table Unit Learning Targets Learning Targets 1.1, 1.5 and 1.12 will be assessed on quizzes, but not the unit test. 1.1 I can write the names and symbols of the elements in columns 1A – 4A on the periodic table. 1.5 I can write the names and symbols of the elements in columns 5A- 8A on the p ...
... Periodic Table Unit Learning Targets Learning Targets 1.1, 1.5 and 1.12 will be assessed on quizzes, but not the unit test. 1.1 I can write the names and symbols of the elements in columns 1A – 4A on the periodic table. 1.5 I can write the names and symbols of the elements in columns 5A- 8A on the p ...
2.1 Elements
... A. Relating Valence Electrons to Group Number • Elements in the same group have similar electron configurations. • Elements in the same group have the same number of valence electrons. ...
... A. Relating Valence Electrons to Group Number • Elements in the same group have similar electron configurations. • Elements in the same group have the same number of valence electrons. ...
Test Review with answer key and explanations
... on the periodic table of elements is calcium. 14. Has the same number of protons and electrons An atom being neutral means that the charge is 0. Since protons have a positive charge and electrons have a negative charge, in order to have a charge of 0, the neutral atom has to have the same number of ...
... on the periodic table of elements is calcium. 14. Has the same number of protons and electrons An atom being neutral means that the charge is 0. Since protons have a positive charge and electrons have a negative charge, in order to have a charge of 0, the neutral atom has to have the same number of ...
Blank Quiz - Fort Bend ISD
... on the periodic table of elements is calcium. 14. Has the same number of protons and electrons An atom being neutral means that the charge is 0. Since protons have a positive charge and electrons have a negative charge, in order to have a charge of 0, the neutral atom has to have the same number of ...
... on the periodic table of elements is calcium. 14. Has the same number of protons and electrons An atom being neutral means that the charge is 0. Since protons have a positive charge and electrons have a negative charge, in order to have a charge of 0, the neutral atom has to have the same number of ...
по темі “Atoms and Molecules. The Periodic Table”
... The crater Mendeleev on the Moon, as well as element number 101, the radioactive Mendelevium, are named after him. VI. Imagine... that you have discovered a new element... What name would you like to give to it? Describe its properties, its molecular weight, its use in chemistry, pharmacy or industr ...
... The crater Mendeleev on the Moon, as well as element number 101, the radioactive Mendelevium, are named after him. VI. Imagine... that you have discovered a new element... What name would you like to give to it? Describe its properties, its molecular weight, its use in chemistry, pharmacy or industr ...
(Neon) - PowerPoint Presentation
... Protons=10 Electrons=10 Neutrons=10 Atomic weight=20 Atomic number=10 Symbol= Ne ...
... Protons=10 Electrons=10 Neutrons=10 Atomic weight=20 Atomic number=10 Symbol= Ne ...
2.4 The Periodic Table
... • The atomic weight is calculated as the sum of the masses of the individual isotopes for that element. Atomic weight = [(isotope abundance) × (isotope mass)] • The Greek symbol indicates summing of terms. ...
... • The atomic weight is calculated as the sum of the masses of the individual isotopes for that element. Atomic weight = [(isotope abundance) × (isotope mass)] • The Greek symbol indicates summing of terms. ...
Chem102_ch03_atoms_and_the_periodic_table
... Definitions Electrons in the highest occupied energy level are the greatest stable distance from the nucleus. These outermost electrons are known as valence electrons. Shell is a principal energy level defined by a given value of n, where n can be 1,2,3,4 etc… and is capable of holding 2n2 electron ...
... Definitions Electrons in the highest occupied energy level are the greatest stable distance from the nucleus. These outermost electrons are known as valence electrons. Shell is a principal energy level defined by a given value of n, where n can be 1,2,3,4 etc… and is capable of holding 2n2 electron ...
Masses of Atoms
... Atomic Mass Unit ~ 1/12th of the mass of one carbon-12 atom The periodic table shows the atomic mass of Nickel as 58.693. How can there be a decimal point, if the mass is whole numbers of protons and neutrons? ...
... Atomic Mass Unit ~ 1/12th of the mass of one carbon-12 atom The periodic table shows the atomic mass of Nickel as 58.693. How can there be a decimal point, if the mass is whole numbers of protons and neutrons? ...
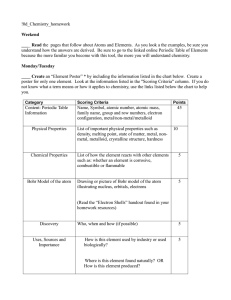
10_Chemistry homework
... 2. The atoms of one element are different from the a toms of another element. 3. Atoms combine in definite ratios to make compounds. 4. Combinations of atoms in compounds can change only when a chemical reaction happens. This means reactions alter atom combinations, but the identity of the atoms the ...
... 2. The atoms of one element are different from the a toms of another element. 3. Atoms combine in definite ratios to make compounds. 4. Combinations of atoms in compounds can change only when a chemical reaction happens. This means reactions alter atom combinations, but the identity of the atoms the ...
18 Chapter 2: The Atom An atom is the smallest particle of an element
... 1912, Danish physicist Niels Henrik David Bohr (1885 – 1962) proposed a new model of the atom. This model is called the “Bohr model” or the “planetary model”. Bohr was interested in the atomi ...
... 1912, Danish physicist Niels Henrik David Bohr (1885 – 1962) proposed a new model of the atom. This model is called the “Bohr model” or the “planetary model”. Bohr was interested in the atomi ...
Nuclide, Atomic Number, mass number - Chemwiki
... The mass of an atom is mostly localized to the nucleus. Because an electron has negligible mass relative to that of a proton or a neutron, the mass number is calculated by the sum of the number of protons and neutrons. Each proton and neutron's mass is approximately one atomic mass unit (AMU). The t ...
... The mass of an atom is mostly localized to the nucleus. Because an electron has negligible mass relative to that of a proton or a neutron, the mass number is calculated by the sum of the number of protons and neutrons. Each proton and neutron's mass is approximately one atomic mass unit (AMU). The t ...
3 chemical foundations: elements, atoms and ions
... 3.3 The Periodic Table of Elements The Periodic Table of Elements is an organisation of atoms according to their properties. It was devised in 1867 by the Russian chemist, Dimitry Mendeleev and independently that same year by the German, Lothar Meyer. Mendeleev gets the title of “Father of Modern Ch ...
... 3.3 The Periodic Table of Elements The Periodic Table of Elements is an organisation of atoms according to their properties. It was devised in 1867 by the Russian chemist, Dimitry Mendeleev and independently that same year by the German, Lothar Meyer. Mendeleev gets the title of “Father of Modern Ch ...
Chapter 2 - Chemistry
... chemical nomenclature - systematic naming of chemical compounds Ionic Compounds - substances composed of ions - positive ion name (metal) is given first followed by the name of the negative ion (nonmetal) Types of ions: i.) monatomic ion - an ion formed from a single atom ii.) polyatomic ion - an io ...
... chemical nomenclature - systematic naming of chemical compounds Ionic Compounds - substances composed of ions - positive ion name (metal) is given first followed by the name of the negative ion (nonmetal) Types of ions: i.) monatomic ion - an ion formed from a single atom ii.) polyatomic ion - an io ...