only that they did. democritus, an early greek philosopher, even had
... Being asked what animal you'd like to be is a trick question; you're already an animal. Doug Coupland ...
... Being asked what animal you'd like to be is a trick question; you're already an animal. Doug Coupland ...
Chapter 2 PowerPoint
... than one type of element are molecular compounds. • Most molecules are composed of nonmetals. • Chemical formulas that indicate actual number and types of atoms in a molecules are called molecular formulas. Such as H2O, C6H12O6, and C2H4. • Empirical formulas give only the relative number of atoms, ...
... than one type of element are molecular compounds. • Most molecules are composed of nonmetals. • Chemical formulas that indicate actual number and types of atoms in a molecules are called molecular formulas. Such as H2O, C6H12O6, and C2H4. • Empirical formulas give only the relative number of atoms, ...
Chapter 5: Atomic Structure
... than one type of element are molecular compounds. • Most molecules are composed of nonmetals. • Chemical formulas that indicate actual number and types of atoms in a molecules are called molecular formulas. Such as H2O, C6H12O6, and C2H4. • Empirical formulas give only the relative number of atoms, ...
... than one type of element are molecular compounds. • Most molecules are composed of nonmetals. • Chemical formulas that indicate actual number and types of atoms in a molecules are called molecular formulas. Such as H2O, C6H12O6, and C2H4. • Empirical formulas give only the relative number of atoms, ...
Element Group Reaction with Oxygen Reaction with Water Lithium
... 9. Mendeleev predicted that ekaaluminium, Ea, and ekasilicon, Es, (the temporary names he gave to the undiscovered elements between zinc and arsenic) would react with oxygen to produce Ea2O3 and EsO2 respectively. On what did he base these predictions? ...
... 9. Mendeleev predicted that ekaaluminium, Ea, and ekasilicon, Es, (the temporary names he gave to the undiscovered elements between zinc and arsenic) would react with oxygen to produce Ea2O3 and EsO2 respectively. On what did he base these predictions? ...
An Overview of Chemistry Lecture 3 Lecture 3
... Compounds • Matter composed two or more different elements that are chemically bound together and do not vary in composition Mixtures • Matter composed two or more different elements or compounds that can vary in their parts by mass. Elements represent one example of a substance, which is matter who ...
... Compounds • Matter composed two or more different elements that are chemically bound together and do not vary in composition Mixtures • Matter composed two or more different elements or compounds that can vary in their parts by mass. Elements represent one example of a substance, which is matter who ...
atoms
... if a negative charge or subtracted from proton number for a positive charge. 4. Neutrons = mass number – protons. 5. Mass number = protons + neutrons 6. Atomic mass= ave mass of all isotopes of that element 7. Atomic symbol- 1 or 2 letter abbreviation for the name of the element. Some from Latin nam ...
... if a negative charge or subtracted from proton number for a positive charge. 4. Neutrons = mass number – protons. 5. Mass number = protons + neutrons 6. Atomic mass= ave mass of all isotopes of that element 7. Atomic symbol- 1 or 2 letter abbreviation for the name of the element. Some from Latin nam ...
SNC1D Periodic Table and Atomic Structure Package
... Unlike the naming of the elements, the system for determining the symbols follows a set of rules. In 1817, the system of chemical symbols that we use today was first proposed by the Swedish chemist Jons Jakob Berzelius (1779-1848). Eventually this system was accepted all around the world. It was a ...
... Unlike the naming of the elements, the system for determining the symbols follows a set of rules. In 1817, the system of chemical symbols that we use today was first proposed by the Swedish chemist Jons Jakob Berzelius (1779-1848). Eventually this system was accepted all around the world. It was a ...
Atoms
... Key Facts about Isotopes • Isotopes: – Atoms of the same element – BUT, Have different numbers of neutrons • Atomic number on periodic table does not change (Same # of Protons) • Atomic Mass (amu) on periodic table does not change (Average of most common isotopes) • Mass number changes (Actual numb ...
... Key Facts about Isotopes • Isotopes: – Atoms of the same element – BUT, Have different numbers of neutrons • Atomic number on periodic table does not change (Same # of Protons) • Atomic Mass (amu) on periodic table does not change (Average of most common isotopes) • Mass number changes (Actual numb ...
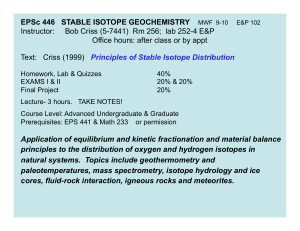
EPSc 446 STABLE ISOTOPE GEOCHEMISTRY Instructor: Bob Criss
... Found that a few α particles were deflected through large angles- up to 180°. ...
... Found that a few α particles were deflected through large angles- up to 180°. ...
MYP 10 PeriodicityWS
... 5(a) Draw a diagram to show the structure of sodium chloride. Explain, in terms of bonding, why sodium chloride has a high melting point. (b) Lithium reacts with water. Write an equation for the reaction and state two observations that could be made during the reaction. [SL paper 2, Nov 05] 6 (a) Fo ...
... 5(a) Draw a diagram to show the structure of sodium chloride. Explain, in terms of bonding, why sodium chloride has a high melting point. (b) Lithium reacts with water. Write an equation for the reaction and state two observations that could be made during the reaction. [SL paper 2, Nov 05] 6 (a) Fo ...
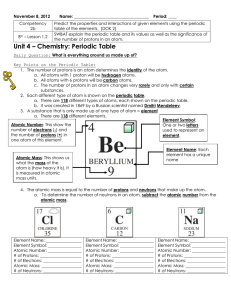
Intro to the Periodic Table
... 7. Find the element with the atomic number of 8. a. What is the element name? _________________________ b. What is the element symbol? _________________________ c. How many protons are in one atom of this element? __________________ d. How many electrons are in one atom of this element? ____________ ...
... 7. Find the element with the atomic number of 8. a. What is the element name? _________________________ b. What is the element symbol? _________________________ c. How many protons are in one atom of this element? __________________ d. How many electrons are in one atom of this element? ____________ ...
Elements
... will simply need to find a way to memorize these. If you notice, all of the halogens fall in this category, and then hydrogen, nitrogen, and oxygen. You will also notice that 2 of these are not gases, make sure you do not for get to include these in your diatomic list. ...
... will simply need to find a way to memorize these. If you notice, all of the halogens fall in this category, and then hydrogen, nitrogen, and oxygen. You will also notice that 2 of these are not gases, make sure you do not for get to include these in your diatomic list. ...
GHW - Louisiana Tech University
... Inquiry Learning) exercise on Chapter 2. Atoms and Elements: Isotopes, Mole and Grams of Substances. Why? Why do we need the concept of isotopes describing an element? How average atomic masse is calculated from isotopic masses? In chemistry, why is the concept of mole central to standard measuremen ...
... Inquiry Learning) exercise on Chapter 2. Atoms and Elements: Isotopes, Mole and Grams of Substances. Why? Why do we need the concept of isotopes describing an element? How average atomic masse is calculated from isotopic masses? In chemistry, why is the concept of mole central to standard measuremen ...
Chapters 19 & 20
... Elements in group 1A through 8A are called representative elements because they display a wide range of physical and chemical properties. Representative elements display the range of possible valence electrons from one in group 1A to eight in group 8A. The valence electrons of representative element ...
... Elements in group 1A through 8A are called representative elements because they display a wide range of physical and chemical properties. Representative elements display the range of possible valence electrons from one in group 1A to eight in group 8A. The valence electrons of representative element ...
Defining the Atom
... masses of atoms to a standard reference isotope. Carbon-12 is the standard reference isotope. Cabon-12 has a mass of exactly 12 atomic mass units. ...
... masses of atoms to a standard reference isotope. Carbon-12 is the standard reference isotope. Cabon-12 has a mass of exactly 12 atomic mass units. ...
Atomic Structure Notes file
... The atoms of an element can differ in mass from each other because they have differing numbers of neutrons. Those with more neutrons will weigh more and be more massive. The atomic mass (often referred to as atomic weight) of an element is calculated by adding together the number of protons and the ...
... The atoms of an element can differ in mass from each other because they have differing numbers of neutrons. Those with more neutrons will weigh more and be more massive. The atomic mass (often referred to as atomic weight) of an element is calculated by adding together the number of protons and the ...
Name Date Class DEFINING THE ATOM Section Review Objectives
... Part B True-False Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 11. The atomic number of an element is the sum of the protons and electrons in an atom of that element. 12. The atomic number of an atom is the total number of protons in an atom of that el ...
... Part B True-False Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 11. The atomic number of an element is the sum of the protons and electrons in an atom of that element. 12. The atomic number of an atom is the total number of protons in an atom of that el ...
Atoms and Their Parts (Subatomic Particles)
... atom. All atoms of the same element have the same number of protons. The number of protons in the nucleus is call the atomic number and again, is unique to each element. A different number of protons would mean you have a different element. Electrons are negatively charged and are located in shell ...
... atom. All atoms of the same element have the same number of protons. The number of protons in the nucleus is call the atomic number and again, is unique to each element. A different number of protons would mean you have a different element. Electrons are negatively charged and are located in shell ...
1 TEST DATE:
... mass ______________________________ of an atom. The number of neutrons in an atom can be found by subtracting the atomic number from the ____________________________ number. The mass of the atom is so small that there is a measure called the atomic _________________________ unit with a symbol of “µ. ...
... mass ______________________________ of an atom. The number of neutrons in an atom can be found by subtracting the atomic number from the ____________________________ number. The mass of the atom is so small that there is a measure called the atomic _________________________ unit with a symbol of “µ. ...
Atoms
... The element antimony (Sb) has naturally occurring isotopes with mass numbers of 121 and 123. The relative abundance and atomic masses are 57.12 % for mass = 120.90 amu, and 47.29% for mass = 122.90 amu. Calculate the atomic mass of antimony. ...
... The element antimony (Sb) has naturally occurring isotopes with mass numbers of 121 and 123. The relative abundance and atomic masses are 57.12 % for mass = 120.90 amu, and 47.29% for mass = 122.90 amu. Calculate the atomic mass of antimony. ...
atomic number
... http://www.youtube.com/watch?v=qQNpucos9wc http://www.youtube.com/watch?v=RIg1Vh7uPyw&list=TL9l iUotc3avVG69w_AB1a0zk9sCfDLWVc&safe=active ...
... http://www.youtube.com/watch?v=qQNpucos9wc http://www.youtube.com/watch?v=RIg1Vh7uPyw&list=TL9l iUotc3avVG69w_AB1a0zk9sCfDLWVc&safe=active ...
File
... X and Z because they have the same atomic number but different masses. 15. Atoms W, X, Y, and Z have the following nuclear compositions. Which two are isotopes? How do you know? ...
... X and Z because they have the same atomic number but different masses. 15. Atoms W, X, Y, and Z have the following nuclear compositions. Which two are isotopes? How do you know? ...