gallagher chapter 41
... charge, and a relative mass of 1 (or 1840 times that of an electron) 5. 1932 – James Chadwick confirmed the existence of the “neutron” – a particle with no charge, but a mass nearly equal to a proton ...
... charge, and a relative mass of 1 (or 1840 times that of an electron) 5. 1932 – James Chadwick confirmed the existence of the “neutron” – a particle with no charge, but a mass nearly equal to a proton ...
CHEMISTRY The Central Science 9th Edition
... -Usually for the simplicity, we represent the elements by symbols, using the initial letter of the name in capital form, starting by the old known elements, so Carbon is represented by the letter C, but Calcium is represented by the symbol Ca and Cobalt by the symbol Co, ……, Nitrogen is represented ...
... -Usually for the simplicity, we represent the elements by symbols, using the initial letter of the name in capital form, starting by the old known elements, so Carbon is represented by the letter C, but Calcium is represented by the symbol Ca and Cobalt by the symbol Co, ……, Nitrogen is represented ...
I. Atoms
... III. Distinguishing Between Atoms • Atomic number: ─ equal to the number of protons in an atom ─ in a neutral atom: # protons = # electrons How to find on the periodic table: the WHOLE number ...
... III. Distinguishing Between Atoms • Atomic number: ─ equal to the number of protons in an atom ─ in a neutral atom: # protons = # electrons How to find on the periodic table: the WHOLE number ...
Net Ionic Equations
... Important because: the binding of atoms results from the transfer or sharing of electrons. ...
... Important because: the binding of atoms results from the transfer or sharing of electrons. ...
1. Structure and Properties of the Atom
... Atoms of different elements can combine with one another in simple (whole number) ratios to form compounds. ...
... Atoms of different elements can combine with one another in simple (whole number) ratios to form compounds. ...
Balancing reaction equations, oxidation state, and reduction
... Important because: the binding of atoms results from the transfer or sharing of electrons. ...
... Important because: the binding of atoms results from the transfer or sharing of electrons. ...
Chapter 2 PPT - Richsingiser.com
... • The order of the elements in the names and formulas of molecular compounds is: • The element farther to the left in the periodic table appears first. • The element closer to the bottom within any group is first. • Hydrogen is first when combined with 6A and 7A elements; it is named second when com ...
... • The order of the elements in the names and formulas of molecular compounds is: • The element farther to the left in the periodic table appears first. • The element closer to the bottom within any group is first. • Hydrogen is first when combined with 6A and 7A elements; it is named second when com ...
Atomic Structure - Tumwater School District
... 2. All atoms of the same elements are identical. Atoms of different elements must be different. 3. Atoms of different elements can be combined together in ratios to form compounds. 4. Chemical reactions occur when atoms are separated, attached, or rearranged. But atoms aren’t changed, only rearrange ...
... 2. All atoms of the same elements are identical. Atoms of different elements must be different. 3. Atoms of different elements can be combined together in ratios to form compounds. 4. Chemical reactions occur when atoms are separated, attached, or rearranged. But atoms aren’t changed, only rearrange ...
Skill Sheet 19-B Chemical Formulas
... Have you ever heard of sodium nitrate? It’s a preservative used in foods like hot dogs. The chemical formula for sodium nitrate is NaNO3. How many types of atoms does this compound contain? You are right if you said three: sodium, nitrogen, and oxygen. The nitrogen and oxygen atoms have a shared-ele ...
... Have you ever heard of sodium nitrate? It’s a preservative used in foods like hot dogs. The chemical formula for sodium nitrate is NaNO3. How many types of atoms does this compound contain? You are right if you said three: sodium, nitrogen, and oxygen. The nitrogen and oxygen atoms have a shared-ele ...
Ch2 Lecture
... A. Relating Valence Electrons to Group Number •Elements in the same group have similar electron configurations. •Elements in the same group have the same number of valence electrons. ...
... A. Relating Valence Electrons to Group Number •Elements in the same group have similar electron configurations. •Elements in the same group have the same number of valence electrons. ...
O usually has oxidation number of -2, except in peroxides where it is
... O usually has oxidation number of -2, except in peroxides where it is assigned -1, and in OF2 where it is assigned a +2 due to the higher electro negativity of F. -In calcium oxide, CaO, oxide ion has a 2- charge. Its oxidation number is -2 -In H2O2, Each O is assigned the oxidation number of -1 5. ...
... O usually has oxidation number of -2, except in peroxides where it is assigned -1, and in OF2 where it is assigned a +2 due to the higher electro negativity of F. -In calcium oxide, CaO, oxide ion has a 2- charge. Its oxidation number is -2 -In H2O2, Each O is assigned the oxidation number of -1 5. ...
9.1 Electron Transfer Reactions
... 1. The sum of the oxidation numbers in a neutral compound is equal to zero 2. The sum of the oxidation numbers in a polyatomic ion is equal to the ion’s overall charge 3. The oxidation number of an element in its native state is zero 4. The oxidation number of a monatomic ion is the same as its char ...
... 1. The sum of the oxidation numbers in a neutral compound is equal to zero 2. The sum of the oxidation numbers in a polyatomic ion is equal to the ion’s overall charge 3. The oxidation number of an element in its native state is zero 4. The oxidation number of a monatomic ion is the same as its char ...
Atoms FlexBook Atoms FlexBook
... Q: Do you know properties of any other elements? For example, what do you know about helium? A: Helium is a gas that has a lower density than air. That’s why helium balloons have to be weighted down so they won’t float away. Q: Living things, like all matter, are made of elements. Do you know which ...
... Q: Do you know properties of any other elements? For example, what do you know about helium? A: Helium is a gas that has a lower density than air. That’s why helium balloons have to be weighted down so they won’t float away. Q: Living things, like all matter, are made of elements. Do you know which ...
chapter5 - MrFoti.com
... Counting the Pieces Atomic Number = number of protons in the nucleus # of protons determines kind of atom (since all protons are alike!) the same as the number of electrons in the neutral atom. Mass Number = the number of protons + neutrons in a particular isotope of that element. These acc ...
... Counting the Pieces Atomic Number = number of protons in the nucleus # of protons determines kind of atom (since all protons are alike!) the same as the number of electrons in the neutral atom. Mass Number = the number of protons + neutrons in a particular isotope of that element. These acc ...
chem 1 TIFF new.indd
... For most atoms the atomic weight is very close to the sum of the protons and neutrons in the nucleus. Both protons and neutrons have an atomic weight of 1 and electrons are so small that they are given almost no weight at all. The number of neutrons for an atom can be calculated by subtracting the n ...
... For most atoms the atomic weight is very close to the sum of the protons and neutrons in the nucleus. Both protons and neutrons have an atomic weight of 1 and electrons are so small that they are given almost no weight at all. The number of neutrons for an atom can be calculated by subtracting the n ...
Chapter 2
... in a chemical reaction. Elements can only be converted into other elements in nuclear reactions. 3. All atoms of an element have the same number of protons and electrons, which determines the chemical behavior of the element. Isotopes of an element differ in the number of neutrons, and thus in mass ...
... in a chemical reaction. Elements can only be converted into other elements in nuclear reactions. 3. All atoms of an element have the same number of protons and electrons, which determines the chemical behavior of the element. Isotopes of an element differ in the number of neutrons, and thus in mass ...
In actual laboratories, isotopes in a sample can be
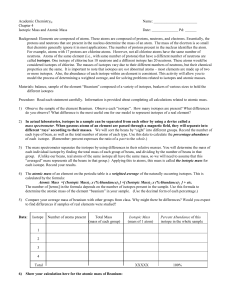
... Background: Elements are composed of atoms. These atoms are composed of protons, neutrons, and electrons. Essentially, the protons and neutrons that are present in the nucleus determine the mass of an atom. The mass of the electron is so small that chemists generally ignore it in most applications. ...
... Background: Elements are composed of atoms. These atoms are composed of protons, neutrons, and electrons. Essentially, the protons and neutrons that are present in the nucleus determine the mass of an atom. The mass of the electron is so small that chemists generally ignore it in most applications. ...
atom - West Ada
... Elements are made of atoms. Each element is made of the same atoms. Change the type of atoms and you change the element. Atoms are composed of a nucleus, which has protons and neutrons, and is surrounded by a dense cloud of electrons. The protons, which are positively charged and the neutrons, whic ...
... Elements are made of atoms. Each element is made of the same atoms. Change the type of atoms and you change the element. Atoms are composed of a nucleus, which has protons and neutrons, and is surrounded by a dense cloud of electrons. The protons, which are positively charged and the neutrons, whic ...
Chapter 2 - WordPress.com
... A. Relating Valence Electrons to Group Number • Elements in the same group have similar electron configurations. • Elements in the same group have the same number of valence electrons. • The group number, 1A–8A, equals the number of valence electrons for the main group elements. • The exception is H ...
... A. Relating Valence Electrons to Group Number • Elements in the same group have similar electron configurations. • Elements in the same group have the same number of valence electrons. • The group number, 1A–8A, equals the number of valence electrons for the main group elements. • The exception is H ...
PowerPoint - De Anza College
... A. Relating Valence Electrons to Group Number • Elements in the same group have similar electron configurations. • Elements in the same group have the same number of valence electrons. ...
... A. Relating Valence Electrons to Group Number • Elements in the same group have similar electron configurations. • Elements in the same group have the same number of valence electrons. ...
Ch 17 Notes
... are the building blocks of matter consists of protons and neutrons in a nucleus surrounded by electrons element-a substance made of only one kind of atom atoms of the same kind make up an element atoms w/ same # of protons belong to same element atoms w/ diff. # of protons are different elements 115 ...
... are the building blocks of matter consists of protons and neutrons in a nucleus surrounded by electrons element-a substance made of only one kind of atom atoms of the same kind make up an element atoms w/ same # of protons belong to same element atoms w/ diff. # of protons are different elements 115 ...
Lecture 1 Medical Chemistry
... Greek prefixes di-, tri-, tetra-, penta-, and hexa- to name them. Thus, the ligands in the cation [Co(NH3)4Cl2]+ are “tetraamminedichloro.” (Note that prefixes are ignored when alphabetizing ligands.) If the ligand itself contains a Greek prefix, we use the prefixes bis (2), tris (3), and tetrakis ( ...
... Greek prefixes di-, tri-, tetra-, penta-, and hexa- to name them. Thus, the ligands in the cation [Co(NH3)4Cl2]+ are “tetraamminedichloro.” (Note that prefixes are ignored when alphabetizing ligands.) If the ligand itself contains a Greek prefix, we use the prefixes bis (2), tris (3), and tetrakis ( ...
2.1 Early Ideas in Atomic Theory
... (a clear and colorless gas, shown here as red spheres) react, their atoms rearrange to form a compound containing copper and oxygen (a powdery, black solid). (credit copper: modification of work by http://imagesof-elements.com/copper.php) ...
... (a clear and colorless gas, shown here as red spheres) react, their atoms rearrange to form a compound containing copper and oxygen (a powdery, black solid). (credit copper: modification of work by http://imagesof-elements.com/copper.php) ...