2 - grade11chemistry
... • Putting all this together, we get B-R diagrams • To draw them you must know the # of protons, neutrons, and electrons (2,8,8,2 filling order) • Draw protons (p+), (n0) in circle (i.e. “nucleus”) • Draw electrons around in shells ...
... • Putting all this together, we get B-R diagrams • To draw them you must know the # of protons, neutrons, and electrons (2,8,8,2 filling order) • Draw protons (p+), (n0) in circle (i.e. “nucleus”) • Draw electrons around in shells ...
Ch#4 Atoms and Elements
... • Compound – distinct substance that is composed of the atoms of two or more elements and always contains exactly the same whole number ratio of those elements. • Chemical Formulas – expresses the types of atoms and the number of each type in each formula unit, or molecule of a given compound. ...
... • Compound – distinct substance that is composed of the atoms of two or more elements and always contains exactly the same whole number ratio of those elements. • Chemical Formulas – expresses the types of atoms and the number of each type in each formula unit, or molecule of a given compound. ...
Atomic structure and periodic table review questions What is an
... 1. What is an atom mostly made up of? 2. The two sub-atomic particles found in the nucleus are? 3. Another name for the two sub-atomic particles found in the nucleus is ____________. 4. What are the sub-atomic particles found outside of the nucleus? 5. Which particle has a positive charge? 6. Which ...
... 1. What is an atom mostly made up of? 2. The two sub-atomic particles found in the nucleus are? 3. Another name for the two sub-atomic particles found in the nucleus is ____________. 4. What are the sub-atomic particles found outside of the nucleus? 5. Which particle has a positive charge? 6. Which ...
Word List
... 1.4 I can describe the charge and location of protons, neutrons, and electrons within the nucleus and shells of an atom. The periodic table is, in many ways, the world’s greatest cheat sheet. The periodic table lists all of the elements (simple substances that make up more complex materials) like go ...
... 1.4 I can describe the charge and location of protons, neutrons, and electrons within the nucleus and shells of an atom. The periodic table is, in many ways, the world’s greatest cheat sheet. The periodic table lists all of the elements (simple substances that make up more complex materials) like go ...
Chapter 3, Section One - Bismarck Public Schools
... Chapter 3, Section One The Atoms Family! What is an Atom? •Ancient Greeks asked the question… –If we were to cut matter (ie a carrot) into smaller and smaller pieces, what would be the smallest piece possible? –The answer? The atom. –Word atom comes from atomos. •Means “to cut” What is an Atom? •Wha ...
... Chapter 3, Section One The Atoms Family! What is an Atom? •Ancient Greeks asked the question… –If we were to cut matter (ie a carrot) into smaller and smaller pieces, what would be the smallest piece possible? –The answer? The atom. –Word atom comes from atomos. •Means “to cut” What is an Atom? •Wha ...
- Lexington JHS
... unknown elements were discovered, it was found that Mendeleev had closely predicted the properties of the elements as well as their discovery. ...
... unknown elements were discovered, it was found that Mendeleev had closely predicted the properties of the elements as well as their discovery. ...
sample
... iron, have been known for thousands of years. Others have been discovered much more recently. Helium, often used in balloons, was discovered in 1895. Americium, used in smoke alarms, was discovered only in 1944. Scientists continue to discover new elements today. The atomic number (proton number) of ...
... iron, have been known for thousands of years. Others have been discovered much more recently. Helium, often used in balloons, was discovered in 1895. Americium, used in smoke alarms, was discovered only in 1944. Scientists continue to discover new elements today. The atomic number (proton number) of ...
Chapter 18 Comparing Atoms Lab
... many marbles, and of as many colors as they need but must take at least as many total marbles as they put in. For example, a player can trade 2 yellows for 1 yellow, 1 blue, and 1 red. 11. Which particles are found in an atom’s nucleus? Which particles are found outside the nucleus? 12. What four el ...
... many marbles, and of as many colors as they need but must take at least as many total marbles as they put in. For example, a player can trade 2 yellows for 1 yellow, 1 blue, and 1 red. 11. Which particles are found in an atom’s nucleus? Which particles are found outside the nucleus? 12. What four el ...
1 - Intro to Electrochemistry
... Its oxidation number _____________________ (more on this later) Example: Cu(s) Cu2+ + 2 eReduction During reduction, a substance ____________ electrons during a chemical reaction The oxidation number of the substance being reduced is ______________ in the ...
... Its oxidation number _____________________ (more on this later) Example: Cu(s) Cu2+ + 2 eReduction During reduction, a substance ____________ electrons during a chemical reaction The oxidation number of the substance being reduced is ______________ in the ...
atoms - Fort Bend ISD
... Materials, when rubbed, can develop a charge difference. This electricity is called “cathode rays” when passed through an evacuated tube (demos). These rays have a small mass and are negative. Thompson noted that these negative subatomic particles were a fundamental part of all atoms. 1) Dalton’s “B ...
... Materials, when rubbed, can develop a charge difference. This electricity is called “cathode rays” when passed through an evacuated tube (demos). These rays have a small mass and are negative. Thompson noted that these negative subatomic particles were a fundamental part of all atoms. 1) Dalton’s “B ...
Topic 2 Part 1 Slides - Coral Gables Senior High
... Subatomic particles are SO SMALL that instead of describing them by their actual values we describe their masses by relative values. That is why these values have no units. Protons and neutrons are relatively the same size, they have a relative mass of 1. Electrons are about 2,000 times smaller tha ...
... Subatomic particles are SO SMALL that instead of describing them by their actual values we describe their masses by relative values. That is why these values have no units. Protons and neutrons are relatively the same size, they have a relative mass of 1. Electrons are about 2,000 times smaller tha ...
Chapters 1-4 Numbers and Measurements in Chemistry Units SI
... • Heaviest atom is ~260 amu (4x10-22 g) • Largest atom is 500 pm across. • Typical C-C bond length 154 pm ...
... • Heaviest atom is ~260 amu (4x10-22 g) • Largest atom is 500 pm across. • Typical C-C bond length 154 pm ...
Multiple Choice - EDU360ScienceMethods
... 13. What evidence indicated that each atom has a nucleus? a. When alpha particles are shot at gold foil some alpha particles are deflected slightly or at large angles. b. Each time an alpha particle hit this zinc sulfide coating, a flash of light was produced at the point of contact. c. Positively ...
... 13. What evidence indicated that each atom has a nucleus? a. When alpha particles are shot at gold foil some alpha particles are deflected slightly or at large angles. b. Each time an alpha particle hit this zinc sulfide coating, a flash of light was produced at the point of contact. c. Positively ...
doc: Oxidation Numbers
... Oxidation Numbers It is often useful to follow chemical reactions by looking at changes in the oxidation numbers of the atoms in each compound during the reaction. Oxidation numbers also play an important role in the naming of chemical compounds. By definition, the oxidation number of an atom is the ...
... Oxidation Numbers It is often useful to follow chemical reactions by looking at changes in the oxidation numbers of the atoms in each compound during the reaction. Oxidation numbers also play an important role in the naming of chemical compounds. By definition, the oxidation number of an atom is the ...
Section 1 Slides - St. John`s College HS
... 1. All elements are composed of tiny invisible particles called atoms. Atoms can be broken down into even smaller, more fundamental particles. ...
... 1. All elements are composed of tiny invisible particles called atoms. Atoms can be broken down into even smaller, more fundamental particles. ...
Isotope
... ATOM that has its orbitals (shell) all full. In order to become full that atom must gain or lose electrons. • Most atoms form compounds in order to be happy. ...
... ATOM that has its orbitals (shell) all full. In order to become full that atom must gain or lose electrons. • Most atoms form compounds in order to be happy. ...
Chapter 4 Atomic Structure
... names of the elements, along with atomic number and average atomic mass ...
... names of the elements, along with atomic number and average atomic mass ...
Atoms, Ions, and Isotopes
... • A negatively charged ion (one that has more electrons than protons) is called an anion. Cl1- ...
... • A negatively charged ion (one that has more electrons than protons) is called an anion. Cl1- ...
- Catalyst
... Question 7: Fill in the blanks of the statements below with the words in the box. Note, you will only use each word once. A. atom ...
... Question 7: Fill in the blanks of the statements below with the words in the box. Note, you will only use each word once. A. atom ...
Note Packet for Students
... Joseph Priestley (1733-1804) discovered oxygen and found to support combustion. He also found carbon dioxide in a fermentation of grain Fundamental Chemical Laws Antoine Lavoisier (1743-1794) described the true reason for combustion. He was very careful when measuring his experimental work and found ...
... Joseph Priestley (1733-1804) discovered oxygen and found to support combustion. He also found carbon dioxide in a fermentation of grain Fundamental Chemical Laws Antoine Lavoisier (1743-1794) described the true reason for combustion. He was very careful when measuring his experimental work and found ...
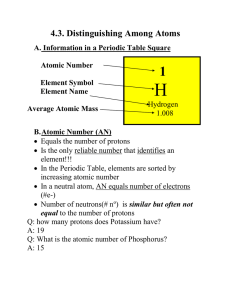
Notes 4.3 filled in
... B. Atomic Number (AN) Equals the number of protons Is the only reliable number that identifies an element!!! In the Periodic Table, elements are sorted by increasing atomic number In a neutral atom, AN equals number of electrons (#e-) Number of neutrons(# no) is similar but often not equal ...
... B. Atomic Number (AN) Equals the number of protons Is the only reliable number that identifies an element!!! In the Periodic Table, elements are sorted by increasing atomic number In a neutral atom, AN equals number of electrons (#e-) Number of neutrons(# no) is similar but often not equal ...
HighFour Chemistry Round 1 Category C: Grades 9 – 10 Thursday
... To balance the 8 H atoms on the right side, 8 moles of HNO3 is required. To finalize the balancing, 2 moles of NO is required to balance out the O and N atoms. The balanced chemical reaction is therefore: ...
... To balance the 8 H atoms on the right side, 8 moles of HNO3 is required. To finalize the balancing, 2 moles of NO is required to balance out the O and N atoms. The balanced chemical reaction is therefore: ...
Definition - kcpe-kcse
... discovery of protons. - by looking at certain trends, among the elements a new organization was created Periodic Law - pattern of repeating properties displayed by elements in the periodic table SO….the periodic table is now arranged by atomic number instead of atomic mass ...
... discovery of protons. - by looking at certain trends, among the elements a new organization was created Periodic Law - pattern of repeating properties displayed by elements in the periodic table SO….the periodic table is now arranged by atomic number instead of atomic mass ...