Electrons
... ionic compound) if electrons were completely transferred to the more electronegative element. 1. Free elements (uncombined state) have an oxidation number of zero. ...
... ionic compound) if electrons were completely transferred to the more electronegative element. 1. Free elements (uncombined state) have an oxidation number of zero. ...
UNIT 2 – THE ATOM - Neshaminy School District
... This is the average of all mass numbers of the element with respect to their frequency in nature. This value is found on the Periodic Table once we know what element the atom is. ...
... This is the average of all mass numbers of the element with respect to their frequency in nature. This value is found on the Periodic Table once we know what element the atom is. ...
ATOMS AND ELEMENTS Evolution of Atomic Theory
... metals.” The classical group numbers for transition metals end in the letter B or have no letter at all (group VIII). ¤ Group IB metals (copper, silver, and gold) are often called the coinage metals. ...
... metals.” The classical group numbers for transition metals end in the letter B or have no letter at all (group VIII). ¤ Group IB metals (copper, silver, and gold) are often called the coinage metals. ...
Activity 2 - SSS Chemistry
... ________________________________________________________________________ ________________________________________________________________________ ...
... ________________________________________________________________________ ________________________________________________________________________ ...
Atomic Mass Lab (Flaskum)
... 10. The atomic mass of chlorine on the periodic table is not a whole number. What does this indicate? What is the most common isotope of chlorine? ...
... 10. The atomic mass of chlorine on the periodic table is not a whole number. What does this indicate? What is the most common isotope of chlorine? ...
8.1 Atoms and Their Parts Assignment
... Electrons are negatively charged and are located in shells or orbits spinning around the nucleus. The number of protons and electrons are usually equal. This equality is important so that the atom as a whole is neither positively nor negatively charged. It is said to be neutral. Electrons have nearl ...
... Electrons are negatively charged and are located in shells or orbits spinning around the nucleus. The number of protons and electrons are usually equal. This equality is important so that the atom as a whole is neither positively nor negatively charged. It is said to be neutral. Electrons have nearl ...
Slide 1
... • the atomic number is usually found at the top of the box for each element in the periodic table. ...
... • the atomic number is usually found at the top of the box for each element in the periodic table. ...
Writing Chemical Formulas
... Do not write a subscript of 1. Reduce the subscripts, if needed. After doing this, be sure the subscripts will not reduce. If both subscripts are divisible by the same number, they must be reduced to have the formula in its proper form. ...
... Do not write a subscript of 1. Reduce the subscripts, if needed. After doing this, be sure the subscripts will not reduce. If both subscripts are divisible by the same number, they must be reduced to have the formula in its proper form. ...
Inside the Atom
... We can calculate age of fossils by calculating how much C14 (radioactive) remains in a sample (accuracy to 35000 years) Uranium also is used to date rocks, however its half life is 4.5 billions years and decays to lead (Pb) scientists calculate age of earth and rocks by comparing amount uranium ...
... We can calculate age of fossils by calculating how much C14 (radioactive) remains in a sample (accuracy to 35000 years) Uranium also is used to date rocks, however its half life is 4.5 billions years and decays to lead (Pb) scientists calculate age of earth and rocks by comparing amount uranium ...
Example of calculating average atomic mass
... 2. Atoms of one element cannot be converted into atoms of another element in a chemical reaction. Elements can only be converted into other elements in nuclear reactions. 3. All atoms of an element have the same number of protons and electrons, which determines the chemical behavior of the element. ...
... 2. Atoms of one element cannot be converted into atoms of another element in a chemical reaction. Elements can only be converted into other elements in nuclear reactions. 3. All atoms of an element have the same number of protons and electrons, which determines the chemical behavior of the element. ...
Periodic Table
... other elements. Besides that, a row goes from left to right, it’s is called a period. Elements of the same period have the same number of electron shells. - Every element in the first column (group one) has one electron in its outer shell. Every element in the second column (group two) has two elect ...
... other elements. Besides that, a row goes from left to right, it’s is called a period. Elements of the same period have the same number of electron shells. - Every element in the first column (group one) has one electron in its outer shell. Every element in the second column (group two) has two elect ...
Element
... •consists of atoms of two or more different elements bound together •can be broken down into a simpler type of matter (elements) by chemical means (but not by physical means) •has properties that are different from its component elements •always contains the same ratio of its component atoms. ...
... •consists of atoms of two or more different elements bound together •can be broken down into a simpler type of matter (elements) by chemical means (but not by physical means) •has properties that are different from its component elements •always contains the same ratio of its component atoms. ...
Chemistry Unit 2: Atomic Structure Unit Assignment #1 1. State the
... 12. How do the three isotopes of hydrogen (H–1, H–2, H–3) compare in terms of the numbers of subatomic particles in each? 13. Write the nuclear symbol for deuterium (H-2): a. Identify the atomic number b. Identify the mass number 14. Determine the number of protons, neutrons, and electrons in Co–59. ...
... 12. How do the three isotopes of hydrogen (H–1, H–2, H–3) compare in terms of the numbers of subatomic particles in each? 13. Write the nuclear symbol for deuterium (H-2): a. Identify the atomic number b. Identify the mass number 14. Determine the number of protons, neutrons, and electrons in Co–59. ...
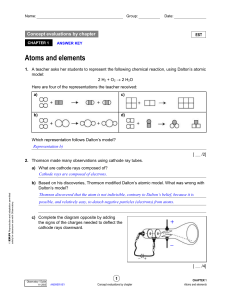
Atoms and Elements
... create gold from base metals and an elixir for everlasting life. Englishman Robert Boyle (1627-1691) is generally credited as the first to study the separate science we call chemistry and the first to perform rigorous experiments. Antoine Lavoisier (1743-1794) discovered the mass of combustion produ ...
... create gold from base metals and an elixir for everlasting life. Englishman Robert Boyle (1627-1691) is generally credited as the first to study the separate science we call chemistry and the first to perform rigorous experiments. Antoine Lavoisier (1743-1794) discovered the mass of combustion produ ...
11129_evl_ch1_ste_corr
... No, they do not all belong to the same period because they do not all have the same number of electron shells. Some of them (boron, nitrogen, fluorine and neon) have two electron shells; others (sodium and magnesium) have three. ...
... No, they do not all belong to the same period because they do not all have the same number of electron shells. Some of them (boron, nitrogen, fluorine and neon) have two electron shells; others (sodium and magnesium) have three. ...
Isotopes - Net Texts
... In Greek, “same place” reads as ίσoςτόπoς (isos topos). This is why atoms which have the same number of protons, but different numbers of neutrons, are called isotopes. They are in the same place on the periodic table! Definition 1: Isotope Isotopes of an element have the same number of protons (sam ...
... In Greek, “same place” reads as ίσoςτόπoς (isos topos). This is why atoms which have the same number of protons, but different numbers of neutrons, are called isotopes. They are in the same place on the periodic table! Definition 1: Isotope Isotopes of an element have the same number of protons (sam ...
1 - WordPress.com
... 40. An ionic bond forms between what types of elements? A metal and a nonmetal An ionic bond is the attraction between positively charged metal cations and negatively charged anions. In an ionic bond, electrons are transferred from the metal (cation) to the nonmetal (anion). What is the structure of ...
... 40. An ionic bond forms between what types of elements? A metal and a nonmetal An ionic bond is the attraction between positively charged metal cations and negatively charged anions. In an ionic bond, electrons are transferred from the metal (cation) to the nonmetal (anion). What is the structure of ...
Document
... • A single compound undergoes a reaction that produces two or more simpler substances. ...
... • A single compound undergoes a reaction that produces two or more simpler substances. ...
the atom
... 2) Atoms of a given element are identical to one another, but different from atoms of any other element. 3) Atoms are rearranged in chemical reactions, but neither the number nor the types of atoms is changed in reaction 4) Compounds are formed by atoms coming together to form molecules in which the ...
... 2) Atoms of a given element are identical to one another, but different from atoms of any other element. 3) Atoms are rearranged in chemical reactions, but neither the number nor the types of atoms is changed in reaction 4) Compounds are formed by atoms coming together to form molecules in which the ...
PreAP Chemistry
... Main Idea: __________________ atoms emit __________________ to gain stability. Radioactivity • __________________ reactions can change one element into another element. • In the late 1890s, scientists noticed some substances __________________ emitted radiation, a process they called _______________ ...
... Main Idea: __________________ atoms emit __________________ to gain stability. Radioactivity • __________________ reactions can change one element into another element. • In the late 1890s, scientists noticed some substances __________________ emitted radiation, a process they called _______________ ...
Mixtures, Pure Substance and Isotopes
... from atomic number of N = 7 7 protons isotope number – atomic number (= 16-7) 9 neutrons number of protons = no. of electrons 7 electrons ...
... from atomic number of N = 7 7 protons isotope number – atomic number (= 16-7) 9 neutrons number of protons = no. of electrons 7 electrons ...
Answer on Question #47967 - Chemistry – Other
... e. A model in which the protons, electrons, and neutrons are evenly distributed throughout the volume of the atom 8. The nucleus of an atom is ____________. a. Positively charged and has a high density b. Negatively charged and has a high density c. Positively charged and has a low density d. Negat ...
... e. A model in which the protons, electrons, and neutrons are evenly distributed throughout the volume of the atom 8. The nucleus of an atom is ____________. a. Positively charged and has a high density b. Negatively charged and has a high density c. Positively charged and has a low density d. Negat ...
Chapter 4 Atomic Structure
... identical. Atoms of any one element are different from those of any other element. ...
... identical. Atoms of any one element are different from those of any other element. ...