PPT_Topic2
... Analysing nuclide notation and drawing diagrams to show electron structure. We will have succeeded if We can draw a diagram and explain important atom numbers to another pupil. ...
... Analysing nuclide notation and drawing diagrams to show electron structure. We will have succeeded if We can draw a diagram and explain important atom numbers to another pupil. ...
Elements and Compounds checklist for web
... Watch Richard Hammond’s ‘metals in a bath tub’ youtube. ...
... Watch Richard Hammond’s ‘metals in a bath tub’ youtube. ...
Agenda/To Do - Perry Local Schools
... your GREEN rubric. 2. Have your Periodic Table out (the one you colored) and your crossword. ...
... your GREEN rubric. 2. Have your Periodic Table out (the one you colored) and your crossword. ...
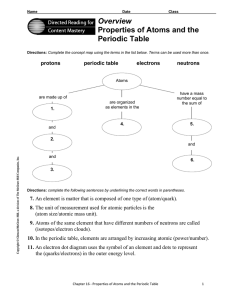
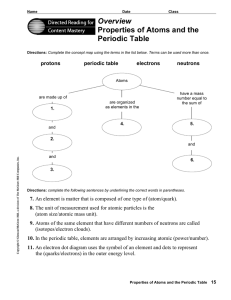
Overview Properties of Atoms and the Periodic Table
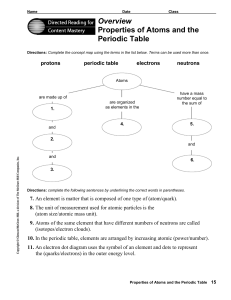
... 8. The unit of measurement used for atomic particles is the (atom size/atomic mass unit). 9. Atoms of the same element that have different numbers of neutrons are called (isotopes/electron clouds). 10. In the periodic table, elements are arranged by increasing atomic (power/number). 11. An electron ...
... 8. The unit of measurement used for atomic particles is the (atom size/atomic mass unit). 9. Atoms of the same element that have different numbers of neutrons are called (isotopes/electron clouds). 10. In the periodic table, elements are arranged by increasing atomic (power/number). 11. An electron ...
Overview Properties of Atoms and the Periodic Table
... . They will also have the same number of ...
... . They will also have the same number of ...
What are Atoms?
... makes them pretty important, and we would do well to understand positively charged nucleus surrounded by a as much about them as we can. But what are elements made of? system of electrons, equal in number to the Based on a knowledge of the way gasses behave, it is clear that number of nuclear proton ...
... makes them pretty important, and we would do well to understand positively charged nucleus surrounded by a as much about them as we can. But what are elements made of? system of electrons, equal in number to the Based on a knowledge of the way gasses behave, it is clear that number of nuclear proton ...
Atoms are not the smallest thing
... effect is balanced by something which causes the space through which the corpuscles are spread to act as if it had a charge of positive electricity equal in amount to the sum of the negative charges of the corpuscles…” ...
... effect is balanced by something which causes the space through which the corpuscles are spread to act as if it had a charge of positive electricity equal in amount to the sum of the negative charges of the corpuscles…” ...
Unit 2 Notes Name - Mr. Walsh`s AP Chemistry
... o Ionic compounds are soluble in water if the sum of all of their attractions to the water molecules is greater than their attraction to each other. A good rule of thumb (though there are exceptions) is that almost all compounds with alkali metal and halogen ions are soluble. Most (but not all) comp ...
... o Ionic compounds are soluble in water if the sum of all of their attractions to the water molecules is greater than their attraction to each other. A good rule of thumb (though there are exceptions) is that almost all compounds with alkali metal and halogen ions are soluble. Most (but not all) comp ...
Study Guide: First Page Which particle defines the element?
... • Mass number. Ex) 26 is the number of protons plus neutrons In the example to the right, why don’t I have to include the number of protons before Magnesium? Ex. ...
... • Mass number. Ex) 26 is the number of protons plus neutrons In the example to the right, why don’t I have to include the number of protons before Magnesium? Ex. ...
PowerPoint Presentation - Introduction to Atoms & Nuclei
... can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. ...
... can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. ...
4 1 introduction to atoms 65-68
... ________________________________________________________________________ 14. How does the mass of an electron compare to the mass of a proton? ________________________________________________________________________ ________________________________________________________________________ 15. An elem ...
... ________________________________________________________________________ 14. How does the mass of an electron compare to the mass of a proton? ________________________________________________________________________ ________________________________________________________________________ 15. An elem ...
Everything around us is made up of atoms. Atoms are one of the
... has. It also tells us the atomic number and atomic mass. Elements are arranged in the periodic table from left to right and top to bottom in order of increasing mass. Each element is identified by an abbreviation (H=Hydrogen, Na=Sodium, K=Potassium, and so on). The table starts with hydrogen (with a ...
... has. It also tells us the atomic number and atomic mass. Elements are arranged in the periodic table from left to right and top to bottom in order of increasing mass. Each element is identified by an abbreviation (H=Hydrogen, Na=Sodium, K=Potassium, and so on). The table starts with hydrogen (with a ...
9 19 -1 atomic number mass number charge
... atomic number of 9, and therefore has 9 protons. All atoms of an element have the same atomic number, by definition. Mass number - This is the total number of nucleons, or protons and neutrons. You can get the number of neutrons by simple subtraction. In this case, the fluorine has 10 neutrons. Atom ...
... atomic number of 9, and therefore has 9 protons. All atoms of an element have the same atomic number, by definition. Mass number - This is the total number of nucleons, or protons and neutrons. You can get the number of neutrons by simple subtraction. In this case, the fluorine has 10 neutrons. Atom ...
PHY–309 L. Solutions for homework set # 10. Textbook question Q
... This wikipedia page shows a diagram of a color CRT tube. In any CRT tube — a TV, a monitor, an oscilloscope, or an X-ray tube — a beam of electrons hits the anode (a screen, or just a piece of metal) at high speed. When the atoms in the anode are hit by fast electrons, sometimes the inner electrons ...
... This wikipedia page shows a diagram of a color CRT tube. In any CRT tube — a TV, a monitor, an oscilloscope, or an X-ray tube — a beam of electrons hits the anode (a screen, or just a piece of metal) at high speed. When the atoms in the anode are hit by fast electrons, sometimes the inner electrons ...
Dalton Model Reading
... chemical process; a chemical reaction simply changes the way atoms are grouped together. 5. Elements are made of tiny particles called atoms. Dalton proposed an additional "rule of greatest simplicity" that created controversy, since it could not be independently confirmed. When atoms combine in onl ...
... chemical process; a chemical reaction simply changes the way atoms are grouped together. 5. Elements are made of tiny particles called atoms. Dalton proposed an additional "rule of greatest simplicity" that created controversy, since it could not be independently confirmed. When atoms combine in onl ...
The periodic table and the atom part 2
... The atomic mass is the average mass of an element in atomic mass units . Though individual atoms always have an integer number of atomic mass units, the atomic mass on the periodic table is stated as a decimal number because it is an average of the various isotopes of an element. The average number ...
... The atomic mass is the average mass of an element in atomic mass units . Though individual atoms always have an integer number of atomic mass units, the atomic mass on the periodic table is stated as a decimal number because it is an average of the various isotopes of an element. The average number ...
9.3 Atoms and Elements notes
... number of electrons in an atom = number of protons Electrons are arranged in energy levels (also known as shells) around the nucleus. The lowest energy levels are always filled first. These are closer to the nucleus and hold the least numbers of electrons. The first energy level can only hold 2 el ...
... number of electrons in an atom = number of protons Electrons are arranged in energy levels (also known as shells) around the nucleus. The lowest energy levels are always filled first. These are closer to the nucleus and hold the least numbers of electrons. The first energy level can only hold 2 el ...
September 20th, 2012
... b) If the atom is assumed to be a sphere, what is the volume in cubic cm of a single Kr atom? ...
... b) If the atom is assumed to be a sphere, what is the volume in cubic cm of a single Kr atom? ...
Atom Reading Passage and Questions File
... The atomic mass (also referred to as the atomic weight) is the sum total of the number of protons and neutrons in an atom. Hydrogen is different from all other atoms in that the hydrogen atom normally does not contain a neutron. The hydrogen atom is composed of one proton and one electron but no neu ...
... The atomic mass (also referred to as the atomic weight) is the sum total of the number of protons and neutrons in an atom. Hydrogen is different from all other atoms in that the hydrogen atom normally does not contain a neutron. The hydrogen atom is composed of one proton and one electron but no neu ...
Discussion Notes (cont.)
... How can atoms of the same element be different? • Isotopes of an element have the same number of protons and electrons but different numbers of neutrons. • The average atomic mass of an element listed in the periodic table is the weighted average mass of the naturally occurring isotopes of that elem ...
... How can atoms of the same element be different? • Isotopes of an element have the same number of protons and electrons but different numbers of neutrons. • The average atomic mass of an element listed in the periodic table is the weighted average mass of the naturally occurring isotopes of that elem ...
LBC1_Sec3_Unit01_Alchemy
... How can atoms of the same element be different? • Isotopes of an element have the same number of protons and electrons but different numbers of neutrons. • The average atomic mass of an element listed in the periodic table is the weighted average mass of the naturally occurring isotopes of that elem ...
... How can atoms of the same element be different? • Isotopes of an element have the same number of protons and electrons but different numbers of neutrons. • The average atomic mass of an element listed in the periodic table is the weighted average mass of the naturally occurring isotopes of that elem ...
the atom
... 2) Atoms of a given element are identical to one another, but different from atoms of any other element. 3) Atoms are rearranged in chemical reactions, but neither the number nor the types of atoms is changed in reaction 4) Compounds are formed by atoms coming together to form molecules in which the ...
... 2) Atoms of a given element are identical to one another, but different from atoms of any other element. 3) Atoms are rearranged in chemical reactions, but neither the number nor the types of atoms is changed in reaction 4) Compounds are formed by atoms coming together to form molecules in which the ...
Atomic Structure Guided Notes
... Changes in the number of particles in the nucleus (protons or neutrons) are ______________ ______________. They only take place in ___________________ processes. Electrons have a mass of almost __________________, which means that the mass of each atom results almost entirely from the number of prot ...
... Changes in the number of particles in the nucleus (protons or neutrons) are ______________ ______________. They only take place in ___________________ processes. Electrons have a mass of almost __________________, which means that the mass of each atom results almost entirely from the number of prot ...
Reading Assignment Worksheet on Atoms - District 196 e
... 7. An element is matter that is composed of one type of (atom/quark). 8. The unit of measurement used for atomic particles is the (atom size/atomic mass unit). 9. Atoms of the same element that have different numbers of neutrons are called (isotopes/electron clouds). 10. In the periodic table, eleme ...
... 7. An element is matter that is composed of one type of (atom/quark). 8. The unit of measurement used for atomic particles is the (atom size/atomic mass unit). 9. Atoms of the same element that have different numbers of neutrons are called (isotopes/electron clouds). 10. In the periodic table, eleme ...
ATOMS AND ELEMENTS Evolution of Atomic Theory
... ! Suppose the atom loses three electrons. ! We symbolize this ion as Al3+. ! Note that losing electrons is indicated with +, and gaining electrons is indicated with -. ...
... ! Suppose the atom loses three electrons. ! We symbolize this ion as Al3+. ! Note that losing electrons is indicated with +, and gaining electrons is indicated with -. ...