* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 3 chemical foundations: elements, atoms and ions
Molecular orbital diagram wikipedia , lookup
Condensed matter physics wikipedia , lookup
Biochemistry wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Oxidation state wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Livermorium wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Atomic orbital wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
Electronegativity wikipedia , lookup
Hydrogen atom wikipedia , lookup
Isotopic labeling wikipedia , lookup
Metallic bonding wikipedia , lookup
Metalloprotein wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Molecular dynamics wikipedia , lookup
Periodic table wikipedia , lookup
Chemical element wikipedia , lookup
Chemical bond wikipedia , lookup
Electron configuration wikipedia , lookup
History of chemistry wikipedia , lookup
Extended periodic table wikipedia , lookup
Atomic nucleus wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
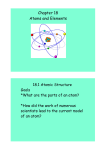
3 CHEMICAL FOUNDATIONS: ELEMENTS, ATOMS AND IONS All matter is built up from chemical combinations of elements. As of 2003, there are 114 known elements, of which 88 are naturally occurring; the remaining 26 elements are synthetic. The term element is used by chemists in several different ways. One way is to refer to atoms of an element. Atoms are the smallest indivisible form of an element. Another way is to refer to the natural chemical state of the element. For example, the element oxygen is found naturally in the microscopic state as a chemical association (molecule) of 2 oxygen atoms. Care must be taken when referring to an element to indicate whether the reference is to an atom or a molecule of the element. 3.1 An Introduction to Atomic Theory The first (relatively) modern theory of the atom was introduced by the English scientist John Dalton in 1808. 3.1.1 DALTON’S ATOMIC THEORY (1808) 1. 2. 3. 4. 5. Elements are made up of tiny indivisible particles called atoms. The atoms of each element are identical. The atoms of any element are different from those of other elements. Atoms of one element combine with atoms of other elements to form compounds. A given compound always has the same relative number and types of atoms. Atoms are neither created nor destroyed in chemical processes. A chemical reaction simply changes the ways atoms are grouped together. Dalton’s atomic theory turned out to be incorrect in its details. However, it did provide a starting point for understanding the nature of matter and of chemical reactions. 3.1.2 THE STRUCTURE OF THE ATOM th By the late 19 century, studies on the interaction of light with matter revealed that the atom has a more complicated structure than Dalton had imagined. 1897: Plum-pudding Model The English scientist J.J. Thomson discovered that atoms contained tiny negatively charged particles that are now referred to as electrons (literally, particles that carry electricity). On the principle that there must be positive charges to counterbalance the negative charges, Thomson postulated that the atom must also contain an overall positive charge of exactly the same magnitude but opposite in sign from the electrons. Lord Kelvin (William Thomson) then proposed that the atom consists of a uniform “pudding” of positive charge with electrons dotted like raisins throughout. 1911: Nuclear Model of the Atom – Ernest Rutherford and Neils Bohr 1909 – Hans Geiger and Ernest Marsden (Rutherford’s students) studied the scattering patterns of -particle off a gold foil. In 1911, Rutherford proposed that: 1. The atom is mostly empty space. 2. Most of the mass and all of the positive charge is concentrated in a small densely packed volume called the nucleus. 3. The electrons are moving around in the (mostly) empty space outside the nucleus. In 1919, Rutherford concluded that the positive charge of the nucleus was provided by particles called protons. In 1932, Rutherford and a co-worker, James Chadwick were able to show that page 25 some atomic nuclei contained small neutrally charged particles called neutrons. Each neutron has approximately the same mass as a proton. 1913 – Neils Bohr, on the basis of experiments on the light emitted by gaseous atoms, proposed the so-called planetary model of the atom. In this model, the electrons do not move at random in the space outside of the nucleus. Rather, they may be found in orbits at fixed distances from their nucleus. Introduction to the Modern Concept of Atomic Structure (1926) For the purposes of chemistry, there are three particles that constitute the atom: 1. electron (e–) – this is the fundamental unit of negative charge (e = –1.602 x 10–19 C); its mass is almost zero (me = 9.109 x 10–31 kg). 2. proton (p+) – this is the fundamental unit of positive charge (e = 1.602 x 10–19 C); its mass is 1.6726 x 10–27 kg. 3. neutron (n0) – this is a fundamental unit carrying no charge; its mass is approximately the same as that of a proton, 1.6749 x 10–27 kg. The electrons move at random outside of the nucleus, although they do occupy preferred regions in space. Although the electrons move at random, they each have a very definite fixed energy. The physical and chemical properties of an atom really depend on the energy, number and arrangement of the electrons in the space outside of the nucleus. When atoms combine to form molecules, the electrons of the different atoms intermingle to hold the nuclei together. 3.2 Isotopes We know that the chemical properties and many of the physical properties of an atom depend on the energy, number and arrangement of its electrons. We also know that the number of protons must equal the number of electrons in a free atom. Thus the character of an atom is defined through its protons and electrons. The number of protons present in the nucleus identifies the atom uniquely as one of a particular element. Unlike the electrons and protons, the neutrons do not contribute to the chemical or most of the physical properties of the atoms. Atoms of an element can have variable number of number of neutrons. Thus, for example, a sample of pure carbon atoms can contain carbon atoms having 6, 7, 8 or more neutrons (see the second-to-last column in the table below). e.g. Symbol Atomic Number Number of Protons Number of electrons Number of neutrons Mass Number Carbon-8 8 6 C 6 6 6 2 8 Carbon-9 9 6 C 6 6 6 3 9 Carbon-10 10 6 C 6 6 6 4 10 Carbon-11 11 6 C 6 6 6 5 11 Carbon-12 12 6 C 6 6 6 6 12 Carbon-13 13 6 C 6 6 6 7 13 Carbon-14 14 6 C 6 6 6 8 14 Carbon-15 15 6 6 6 6 9 15 Carbon-20 20 6 6 6 6 14 20 Name C C page 26 Note that all of these atoms are equally as “carbon” as any other (although the carbon atom having 6 neutrons is by far the most abundant). These different types of atoms of carbon, having different numbers of neutrons are called isotopes. The conventional notation for writing isotopes is as follows: ASy Z Here, Sy = the one to two letter symbol for the atom; Z = the atomic number of the atom (the number of protons, also equalling the number of electrons in the free atom); and A = the mass number of the isotope. For each type of atom of an element, Sy and Z are always the same. The mass number, A, can vary depending on the number of neutrons present in the isotope. A equals the sum of the number of protons (Z) and the number of neutrons (Nn). A = Z + Nn 3.3 The Periodic Table of Elements The Periodic Table of Elements is an organisation of atoms according to their properties. It was devised in 1867 by the Russian chemist, Dimitry Mendeleev and independently that same year by the German, Lothar Meyer. Mendeleev gets the title of “Father of Modern Chemistry” because Meyer did not make any further use of his table. Mendeleev (and Meyer) noted that many of the known elements have very similar physical and chemical properties. Those atoms with such similar properties were put in columns. Thus, for example, atoms such as Na and K were soft, silvery, greyish metals which reacted explosively with water to form extremely strong alkaline solutions. These were placed in a column, called a group in the Periodic Table. This group is known as the alkali metals. Other metals such as Mg and Ca found in the ground also formed alkaline solutions with water, but not in nearly the same explosive fashion as Na and K. These metals were known as the alkaline-earth metals. This arrangement of atoms according to their properties soon proved its value. Gaps in Mendeelev’s Table soon began to appear, and he was able to predict the existence of as yet unknown elements. Chemists began to search for these “missing” elements and found several of them. The modern Periodic Table has three basic characteristics that provide information about the atom. The example of the carbon atom is shown below. Atomic Number (number of protons and electrons present in the free atom) Mass Number (the average mass of a carbon atom in atomic mass units) 6 C Symbol of the atom 12.011 The elements may be classified according to whether they are metals, nonmetals or metalloids (or semimetals). Students are expected to know the name and chemical symbol of the following elements (listed by atomic number): 1 – 38, 40, 42, 46 – 51, 53 – 57, 74, 78 – 80, 82. page 27 3.3.1 METALS The majority of the elements are metals. Metals have the following characteristics. They are: • • • • Lustrous (shiny) Malleability (they can be hammered into thin sheets) Ductile (they can be drawn out into thin wires) Good conductors of heat and electricity The only metal which is not a solid at room temperature and normal atmospheric pressure is mercury, Hg, which is a liquid under these conditions. 3.3.2 NONMETALS Nonmetals lack the properties that characterise metals. They also show a wider variation in their properties. Thus some nonmetals such as carbon are solid, others, like oxygen are gases and one nonmetalllic element, bromine, is liquid. The nonmetals are found to the right of the Periodic Table, past the “step-line” beginning between the atoms B and Al. 3.3.3 METALLOIDS Elements lying near to the step-line are known as metalloids or semimetals, because their properties are intermediate between the metals and nonmetals. They have a mixture of metallic and nonmetallic properties. 3.4 Natural States of the Elements Matter almost always is found as mixtures of different chemical compounds. Elements are rarely found in their pure forms. However, some elements are relatively unreactive and, on occasion, may be found in pure form. Examples include: the noble metals such as gold (Au), silver (Ag) and platinum (Pt) the noble gases (Group 8) consists of He, Ne, Ar, Kr, Xe The noble gases are single atom elements. That is, the element is the same as the atom. On the other hand, some other elements are chemical combinations of two atoms of the same element. These are the diatomic elements and include hydrogen (H2), nitrogen (N2), oxygen (O2), fluorine (F2), chlorine(Cl2), bromine (Br2) and iodine(I2). Note that of these seven diatomic elements, five are gases. Of the remaining two diatomic elements, one is a liquid (Br2) and the other is a solid (I2). The list of diatomic elements must be memorized. The element sulphur, S, is found as a ring structure consisting if 8 S-atoms. Its correct designation, S8, is not often used; usually as shorthand the symbol S is (incorrectly) used. The atom symbol shorthand is commonly used for all of the metal atoms. There are only two elements that are liquid in their natural state at 25°C and normal atmospheric pressures: bromine (Br2) and mercury (Hg). Some elements may be found in different forms. When this occurs, the different forms are known as allotropes. Thus, for example, the element carbon has three known allotropes: graphite (the stablest form of carbon is greyish and forms sheets), diamond (hard, crystallline and colourless) and buckminsterfullerene (a spherical molecule consisting of alternating five- and six- member rings of carbon atoms; the shape is much like a soccer ball). page 28 3.5 Formulas of Compounds A compound is a pure substance that: 1. composed of atoms of two or more different elements; and 2. always has the same relative masses of those elements. The chemical formula of a compound is most conveniently expressed by the number and type of each atom present. Thus the compound water, a chemical combination of two atoms of hydrogen with one atom of oxygen, can be written as follows: The compound consists of two types of atoms: hydrogen and oxygen H2O The subscript “2” following the “H” indicates that hydrogen contributes two atoms to the compound. Exercise: For the following compounds, state the number of each type of atom present. 6. Dinitrogen monoxide: 7. Aluminium chloride: 8. Carbon monoxide: 9. Carbon tetrachloride: 10. Sulphur trioxide: 11. Dinitrogen pentoxide: 12. Periodic acid: 13. Nitric acid: 14. Potassium dichromate: 15. Glucose: Exercise: Write the formulas for the following compound. 1. A compound consists of one atom of carbon and four atoms of hydrogen. 2. A compound consists of one atom of silicon and two atoms of oxygen. 3. A compound consists of two atoms of nitrogen and five atoms of oxygen 4. A compound consists of two atoms of carbon, two atoms of hydrogen and two atoms of chlorine. 5. A compound consists of two atoms of hydrogen, one atom of phosphorous and three atoms of oxygen. page 29