The Mole - My CCSD
... The Gold Foil Experiment – the atom was not a hard sphere but – was mostly space, with a small concentration of positively-charged mass (the nucleus). ...
... The Gold Foil Experiment – the atom was not a hard sphere but – was mostly space, with a small concentration of positively-charged mass (the nucleus). ...
Atomic Structure Study Guide
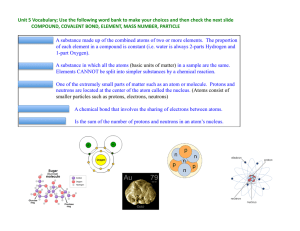
... Isotopes are atoms of the same element that have different numbers of neutrons and different mass numbers. Isotopes of an element have the same atomic number but different mass numbers because they have different numbers of neutrons. Example: every atom of oxygen has 8 protons. Some oxygen ato ...
... Isotopes are atoms of the same element that have different numbers of neutrons and different mass numbers. Isotopes of an element have the same atomic number but different mass numbers because they have different numbers of neutrons. Example: every atom of oxygen has 8 protons. Some oxygen ato ...
How many protons, electrons and neutrons are in an atom of krypton
... charged electrons. Atoms must have equal numbers of protons and electrons. In our example, an atom of krypton must contain 36 electrons since it contains 36 protons. Electrons are arranged around atoms in a special way. If you need to know how the electrons are arranged around an atom, take a look ...
... charged electrons. Atoms must have equal numbers of protons and electrons. In our example, an atom of krypton must contain 36 electrons since it contains 36 protons. Electrons are arranged around atoms in a special way. If you need to know how the electrons are arranged around an atom, take a look ...
Ch 11 HW
... 2. All atoms of the same element contain the same number of __________________ 4. The _________________________ of an element is the number of protons and neutrons in the nucleus. 5. The __________________________ is an average of the masses of all naturally occurring isotopes of an element. ...
... 2. All atoms of the same element contain the same number of __________________ 4. The _________________________ of an element is the number of protons and neutrons in the nucleus. 5. The __________________________ is an average of the masses of all naturally occurring isotopes of an element. ...
Catalyst
... He pounded up materials in his pestle and mortar until he had reduced them to smaller ...
... He pounded up materials in his pestle and mortar until he had reduced them to smaller ...
Atoms: The Building Blocks of Matter
... Dalton’s Atomic Theory - 1808 1. All matter is composed of tiny indivisible particles called atoms 2. Atoms of a given element are identical. Atoms of different elements differ in some ...
... Dalton’s Atomic Theory - 1808 1. All matter is composed of tiny indivisible particles called atoms 2. Atoms of a given element are identical. Atoms of different elements differ in some ...
Unit 2 Complete 2016 2017
... 6)______________________ The one element that has an isotope that does not contain all of the subatomic particles.(Hint: lightest element) 7)______________________ Whenever two elements form more than one compound, the different masses of one element that combine with the same mass of the other elem ...
... 6)______________________ The one element that has an isotope that does not contain all of the subatomic particles.(Hint: lightest element) 7)______________________ Whenever two elements form more than one compound, the different masses of one element that combine with the same mass of the other elem ...
atom - West Ada
... the universe, makes up almost 90% of the total mass of the universe. However, hydrogen atoms make up only about 1% of the Earth’s crust, and most of those hydrogen atoms are combined with oxygen atoms in the form of water. The living things on Earth are composed mostly of the elements oxygen, carbon ...
... the universe, makes up almost 90% of the total mass of the universe. However, hydrogen atoms make up only about 1% of the Earth’s crust, and most of those hydrogen atoms are combined with oxygen atoms in the form of water. The living things on Earth are composed mostly of the elements oxygen, carbon ...
Unit 3 – Atomic Theory
... (fission = splitting). In this reaction, certain specific elements have their nuclei broken down into smaller parts. This reaction releases a tremendous amount of energy, which can be used for an explosion (nuclear weaponry), or to power and electric generator (nuclear reactor). ...
... (fission = splitting). In this reaction, certain specific elements have their nuclei broken down into smaller parts. This reaction releases a tremendous amount of energy, which can be used for an explosion (nuclear weaponry), or to power and electric generator (nuclear reactor). ...
Review for Unit 2A Test
... ___Egypt____, and the __Middle__ __East___. Western civilization traces its roots to __Greece__. The _Roman______ empire "took over from" or "built on" the Greek empire and peaked around the year ___0____. The ___Dark___ Ages began after this empire collapsed and lasted from approximately 500AD to a ...
... ___Egypt____, and the __Middle__ __East___. Western civilization traces its roots to __Greece__. The _Roman______ empire "took over from" or "built on" the Greek empire and peaked around the year ___0____. The ___Dark___ Ages began after this empire collapsed and lasted from approximately 500AD to a ...
Chemistry –Worksheet: Atomic structure
... 18. The most common form of iron has 26 protons and 30 neutrons in its nucleus. State its atomic number, atomic mass, and number of electrons if it's electrically neutral. Atomic number: _______ Atomic mass: ________ # of electrons: __________ 19. Consider the following three atoms: Atom 1 has 7 pro ...
... 18. The most common form of iron has 26 protons and 30 neutrons in its nucleus. State its atomic number, atomic mass, and number of electrons if it's electrically neutral. Atomic number: _______ Atomic mass: ________ # of electrons: __________ 19. Consider the following three atoms: Atom 1 has 7 pro ...
Review Unit 5
... CHEMICALLY STABLE: Elements that are nonreactive because their last electron shell is completely filled with 8 electrons. (e.g. Neon, Argon, Krypton.) ISOTOPE: ...
... CHEMICALLY STABLE: Elements that are nonreactive because their last electron shell is completely filled with 8 electrons. (e.g. Neon, Argon, Krypton.) ISOTOPE: ...
An understanding of the nature of matter has developed
... reportedly synthesized, with elements 113, 115, 117, and 118 having yet to be confirmed. The first 98 elements exist naturally although some are found only in trace amounts and were synthesized in laboratories before being found in nature.[n 1] Elements with atomic numbers from 99 to 118 have only b ...
... reportedly synthesized, with elements 113, 115, 117, and 118 having yet to be confirmed. The first 98 elements exist naturally although some are found only in trace amounts and were synthesized in laboratories before being found in nature.[n 1] Elements with atomic numbers from 99 to 118 have only b ...
Physical Science Chapter 1
... elements are not abbreviations of their English names. Silver is Ag for argentum. Sodium is Na for natrium. Potassium is K for kalium. Antimony is Sb for stibium and Tungsten is W for wolfram. ...
... elements are not abbreviations of their English names. Silver is Ag for argentum. Sodium is Na for natrium. Potassium is K for kalium. Antimony is Sb for stibium and Tungsten is W for wolfram. ...
Atomic Structure Problem Set PROBLEM SET #3: ATOMIC
... Base your answer to the question on the information and the bright-line spectra represented below. Many advertising signs depend on the production of light emissions from gas-filled glass tubes that are subjected to a high-voltage source. When light emissions are passed through a spectroscope, brigh ...
... Base your answer to the question on the information and the bright-line spectra represented below. Many advertising signs depend on the production of light emissions from gas-filled glass tubes that are subjected to a high-voltage source. When light emissions are passed through a spectroscope, brigh ...
Name ____ Date
... 2. Summarize the major experimental evidence that led to the development of various atomic models, both historic and current. 3. Discriminate between the relative size, charge, position and number of protons, neutrons, and electrons in the atoms of different elements. 4. Correlate atomic structure a ...
... 2. Summarize the major experimental evidence that led to the development of various atomic models, both historic and current. 3. Discriminate between the relative size, charge, position and number of protons, neutrons, and electrons in the atoms of different elements. 4. Correlate atomic structure a ...
Big Science from the Small World of Atom
... periodic table, how the number of valence electrons is correlated with group number in the periodic table. Valence electrons are responsible for chemical bonding (chemical reaction), while core electrons can be used to identify elements. With 3D visualization of X-ray generation, students will be de ...
... periodic table, how the number of valence electrons is correlated with group number in the periodic table. Valence electrons are responsible for chemical bonding (chemical reaction), while core electrons can be used to identify elements. With 3D visualization of X-ray generation, students will be de ...
atom
... All matter is composed of extremely small particles called atoms. Atoms of a given element are identical in size, mass, and other properties; atoms of different elements differ in size, mass, and other properties. Atoms cannot be subdivided, created, or destroyed. Atoms of different elements combine ...
... All matter is composed of extremely small particles called atoms. Atoms of a given element are identical in size, mass, and other properties; atoms of different elements differ in size, mass, and other properties. Atoms cannot be subdivided, created, or destroyed. Atoms of different elements combine ...
Atomic Theory: History of the Atom
... experimental observations that led scientists to postulate the existence of the atom (smallest bit of an element). ...
... experimental observations that led scientists to postulate the existence of the atom (smallest bit of an element). ...
18 Chapter 2: The Atom An atom is the smallest particle of an element
... Proton – (symbol p+) a tiny subatomic particle having a positive electric charge, located in the center of the atom (nucleus). The electron and proton have equal sized, but opposite polarity, electric ...
... Proton – (symbol p+) a tiny subatomic particle having a positive electric charge, located in the center of the atom (nucleus). The electron and proton have equal sized, but opposite polarity, electric ...
Building Atoms - Community Science Workshop Network
... as Au for gold. The number above the name is the atomic number which tells us how many and protons are in an atom of the element. Atoms are neutrally charged, so for every positively charged ...
... as Au for gold. The number above the name is the atomic number which tells us how many and protons are in an atom of the element. Atoms are neutrally charged, so for every positively charged ...
Atoms - Issaquah Connect
... • ALL atoms of the same element have the same number of protons. • All neutral atoms have no overall (net) charge, so … have the same number of electrons as protons • BUT… they can have different numbers of neutrons These are called isotopes of carbon ...
... • ALL atoms of the same element have the same number of protons. • All neutral atoms have no overall (net) charge, so … have the same number of electrons as protons • BUT… they can have different numbers of neutrons These are called isotopes of carbon ...
Atoms - Issaquah Connect
... • ALL atoms of the same element have the same number of protons. • All neutral atoms have no overall (net) charge, so … have the same number of electrons as protons • BUT… they can have different numbers of neutrons These are called isotopes of carbon ...
... • ALL atoms of the same element have the same number of protons. • All neutral atoms have no overall (net) charge, so … have the same number of electrons as protons • BUT… they can have different numbers of neutrons These are called isotopes of carbon ...
Promethium

Promethium, originally prometheum, is a chemical element with symbol Pm and atomic number 61. All of its isotopes are radioactive; it is one of only two such elements that are followed in the periodic table by elements with stable forms, a distinction shared with technetium. Chemically, promethium is a lanthanide, which forms salts when combined with other elements. Promethium shows only one stable oxidation state of +3; however, a few +2 compounds may exist.In 1902, Bohuslav Brauner suggested there was an element with properties intermediate between those of the known elements neodymium (60) and samarium (62); this was confirmed in 1914 by Henry Moseley who, having measured the atomic numbers of all the elements then known, found there was an element with atomic number 61. In 1926, an Italian and an American group claimed to have isolated a sample of element 61; both ""discoveries"" were soon proven to be false. In 1938, during a nuclear experiment conducted at Ohio State University, a few radioactive nuclides were produced that certainly were not radioisotopes of neodymium or samarium, but there was a lack of chemical proof that element 61 was produced, and the discovery was not generally recognized. Promethium was first produced and characterized at Oak Ridge National Laboratory in 1945 by the separation and analysis of the fission products of uranium fuel irradiated in a graphite reactor. The discoverers proposed the name ""prometheum"" (the spelling was subsequently changed), derived from Prometheus, the Titan in Greek mythology who stole fire from Mount Olympus and brought it down to humans, to symbolize ""both the daring and the possible misuse of mankind's intellect"". However, a sample of the metal was made only in 1963.There are two possible sources for natural promethium: rare decays of natural europium-151 (producing promethium-147), and uranium (various isotopes). Practical applications exist only for chemical compounds of promethium-147, which are used in luminous paint, atomic batteries, and thickness measurement devices, even though promethium-145 is the most stable promethium isotope. Because natural promethium is exceedingly scarce, it is typically synthesized by bombarding uranium-235 (enriched uranium) with thermal neutrons to produce promethium-147.