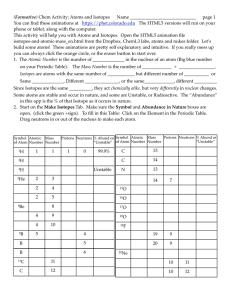
The Structure of the Atom 1 Philosophers And Early Scientists
... First ―chemist‖ to perform quantitative experiments Measured relationship between pressure and volume of air Substance is an element if it can’t be broken down into 2+ simpler substances ...
... First ―chemist‖ to perform quantitative experiments Measured relationship between pressure and volume of air Substance is an element if it can’t be broken down into 2+ simpler substances ...
September 22 Bellwork
... Formula: electrons = protons – charge e = 8 – (-2) Write the ion as either O2- or O-2 ...
... Formula: electrons = protons – charge e = 8 – (-2) Write the ion as either O2- or O-2 ...
Matching - hrsbstaff.ednet.ns.ca
... a. Protons, electrons, and neutrons are evenly distributed throughout the volume of the atom. b. The nucleus is made of protons, electrons, and neutrons. c. Electrons are distributed around the nucleus and occupy almost all the volume of the atom. d. The nucleus is made of electrons and protons. ___ ...
... a. Protons, electrons, and neutrons are evenly distributed throughout the volume of the atom. b. The nucleus is made of protons, electrons, and neutrons. c. Electrons are distributed around the nucleus and occupy almost all the volume of the atom. d. The nucleus is made of electrons and protons. ___ ...
File - Cynthia Campbell
... Ninety-four are found naturally on Earth, and the rest are synthetic elements that have been produced artificially in particle accelerators. Elements 43 (technetium), 61 (promethium) and all elements greater than 83 (bismuth), beginning with 84 (polonium) have no stable isotopes. The atomic mass of ...
... Ninety-four are found naturally on Earth, and the rest are synthetic elements that have been produced artificially in particle accelerators. Elements 43 (technetium), 61 (promethium) and all elements greater than 83 (bismuth), beginning with 84 (polonium) have no stable isotopes. The atomic mass of ...
Chemistry Chapter 4 (Due October 24) [Test
... b. atoms of an element can have different numbers of protons c. atoms are divisible d. all atoms of an element are not identical but they must all have the same mass ____ 16. Why did J. J. Thomson reason that electrons must be a part of the atoms of all elements? a. Cathode rays are negatively-charg ...
... b. atoms of an element can have different numbers of protons c. atoms are divisible d. all atoms of an element are not identical but they must all have the same mass ____ 16. Why did J. J. Thomson reason that electrons must be a part of the atoms of all elements? a. Cathode rays are negatively-charg ...
Chapter # 4 notes
... Thompson showed that atoms contained both positive and negative charges. This disproved the Dalton model of the atom which held that atoms were indivisible. But the mass of the atom could not be accounted for by the mass of protons inside it. There had to be something else. Subatomic Particles: Prot ...
... Thompson showed that atoms contained both positive and negative charges. This disproved the Dalton model of the atom which held that atoms were indivisible. But the mass of the atom could not be accounted for by the mass of protons inside it. There had to be something else. Subatomic Particles: Prot ...
I. Atoms
... • Dalton’s Atomic Theory: developed ~ 1800; based on experiments 1. Each element is composed of tiny *indivisible* particles called atoms. 2. All atoms of a given element are *identical.* Atoms of different elements are unique. 3. Atoms of different elements combine in simple whole number ratios to ...
... • Dalton’s Atomic Theory: developed ~ 1800; based on experiments 1. Each element is composed of tiny *indivisible* particles called atoms. 2. All atoms of a given element are *identical.* Atoms of different elements are unique. 3. Atoms of different elements combine in simple whole number ratios to ...
What is Chemistry? Chemistry
... o Atoms that gain electrons to form compounds are called anions. Anions have a _________________________________. o Naming Anions: Drop the last few letters of the element name and add “ide”. o E.g. Group 17 (Halogens) gain electrons easily and release lots of energy in the process highly reactive ...
... o Atoms that gain electrons to form compounds are called anions. Anions have a _________________________________. o Naming Anions: Drop the last few letters of the element name and add “ide”. o E.g. Group 17 (Halogens) gain electrons easily and release lots of energy in the process highly reactive ...
File
... Of course, since C-14 normally consists of 1/1,000,000,000 of the carbon found in nature, the measurements can be difficult to make and certainly aren't exact. Cobalt-60 in Medical Equipment Sterilization and Food Preservation Cobalt-60, an isotope of cobalt that has one additional neutron in its nu ...
... Of course, since C-14 normally consists of 1/1,000,000,000 of the carbon found in nature, the measurements can be difficult to make and certainly aren't exact. Cobalt-60 in Medical Equipment Sterilization and Food Preservation Cobalt-60, an isotope of cobalt that has one additional neutron in its nu ...
Chemistry I Accelerated StudyGuideline
... More than 2000 years ago, a Greek philosopher named _____________ proposed the existence of very small, indivisible particles, each of which was called a(n) _____________. The theory that such particles existed was supported much later, by _____________ who proposed, in his law of _______________ __ ...
... More than 2000 years ago, a Greek philosopher named _____________ proposed the existence of very small, indivisible particles, each of which was called a(n) _____________. The theory that such particles existed was supported much later, by _____________ who proposed, in his law of _______________ __ ...
Ch. 5 Atomic Structure
... different numbers of neutrons are ________ of the same element. Since isotopes have different numbers of neutrons, they have a different _______ ______. ...
... different numbers of neutrons are ________ of the same element. Since isotopes have different numbers of neutrons, they have a different _______ ______. ...
Chapter 4 Review Packet Section 4.1
... According to the prevailing theory, the alpha particles should have passed easily through the gold, with only a slight deflection due to the positive charge thought to be spread out in the gold atoms. Rutherford’s results were that most alpha particles went straight through, or were slightly deflect ...
... According to the prevailing theory, the alpha particles should have passed easily through the gold, with only a slight deflection due to the positive charge thought to be spread out in the gold atoms. Rutherford’s results were that most alpha particles went straight through, or were slightly deflect ...
Ch. 5 Atomic Structure
... different numbers of neutrons are ________ of the same element. Since isotopes have different numbers of neutrons, they have a different _______ ______. ...
... different numbers of neutrons are ________ of the same element. Since isotopes have different numbers of neutrons, they have a different _______ ______. ...
Here are the answers and work for your summer packet.
... a. An orange liquid is distilled, resulting in the collection of a yellow liquid and a red solid. b. A colorless, crystalline solid is decomposed, yielding a pale yellow-green gas and a soft, shiny metal. c. A cup of tea becomes sweeter as sugar is added to it. a. physical, mixture b. chemical, comp ...
... a. An orange liquid is distilled, resulting in the collection of a yellow liquid and a red solid. b. A colorless, crystalline solid is decomposed, yielding a pale yellow-green gas and a soft, shiny metal. c. A cup of tea becomes sweeter as sugar is added to it. a. physical, mixture b. chemical, comp ...
AP Chemistry Summer Packet ANSWERS
... a. An orange liquid is distilled, resulting in the collection of a yellow liquid and a red solid. b. A colorless, crystalline solid is decomposed, yielding a pale yellow-green gas and a soft, shiny metal. c. A cup of tea becomes sweeter as sugar is added to it. a. physical, mixture b. chemical, comp ...
... a. An orange liquid is distilled, resulting in the collection of a yellow liquid and a red solid. b. A colorless, crystalline solid is decomposed, yielding a pale yellow-green gas and a soft, shiny metal. c. A cup of tea becomes sweeter as sugar is added to it. a. physical, mixture b. chemical, comp ...
Chapter 2 Atoms, Elements, Orbitals, and Electron Configurations
... Questions about the fundamental nature of matter can be traced as far back as the Greek philosophers. • Aristotle believed that matter could be divided indefinitely. • Democritus argued that there was a limit. John Dalton proposed atomic theory in 1808. − The work atom literally means "uncuttable." ...
... Questions about the fundamental nature of matter can be traced as far back as the Greek philosophers. • Aristotle believed that matter could be divided indefinitely. • Democritus argued that there was a limit. John Dalton proposed atomic theory in 1808. − The work atom literally means "uncuttable." ...
Atoms and nukes packet 2016
... 3. So Write this General Rule: If there is only one stable isotope of an element, its Average Atomic mass is : ...
... 3. So Write this General Rule: If there is only one stable isotope of an element, its Average Atomic mass is : ...
ch03 - earthjay science
... Permian System (34): Division of Paleozoic covering geologic time ranging from 286 to 245 million years ago. Named for a Russian province. Fossils were determined to be intermediate between those of Carboniferous below and Triassic above. Phanerozoic Eon (30): The eon of geologic time during which ...
... Permian System (34): Division of Paleozoic covering geologic time ranging from 286 to 245 million years ago. Named for a Russian province. Fossils were determined to be intermediate between those of Carboniferous below and Triassic above. Phanerozoic Eon (30): The eon of geologic time during which ...
Jeopardy - SchoolRack
... $400 Answer from Model of the Atom Atomic number – the number of protons in an atom Atomic Mass – the mass of the protons and neutrons Mass Number – the number of protons and neutrons ...
... $400 Answer from Model of the Atom Atomic number – the number of protons in an atom Atomic Mass – the mass of the protons and neutrons Mass Number – the number of protons and neutrons ...
average atomic mass
... Isotopes have mass numbers Isotopes are atoms of the same element (the same number of p+ and e-) but with different masses due to having different numbers of n0. ...
... Isotopes have mass numbers Isotopes are atoms of the same element (the same number of p+ and e-) but with different masses due to having different numbers of n0. ...
Unit Nuclear Chemistry
... › Emission of subatomic particles or high-energy electromagnetic radiation by nuclei › Such atoms/isotopes said to be radioactive › All nuclides of elements beyond Bismuth (#83) in the periodic table are radioactive with only Polonium (84), Radon (86), Actinium ((89), Thorium (90), Uranium (92) and ...
... › Emission of subatomic particles or high-energy electromagnetic radiation by nuclei › Such atoms/isotopes said to be radioactive › All nuclides of elements beyond Bismuth (#83) in the periodic table are radioactive with only Polonium (84), Radon (86), Actinium ((89), Thorium (90), Uranium (92) and ...
Using your periodic table ppt (9/26-10/11) File
... orbitals & number of valence e-, & to know that properties are similar for elements in a group • Describe the structure of atoms, including the masses, electrical charges, and locations of protons, neutrons, and electrons • Identify that protons determine an elements identity and valence electrons d ...
... orbitals & number of valence e-, & to know that properties are similar for elements in a group • Describe the structure of atoms, including the masses, electrical charges, and locations of protons, neutrons, and electrons • Identify that protons determine an elements identity and valence electrons d ...
Atom - Malibu High School
... Nucleus: the center of the atom; composed of neutrons and protons. Because the mass of the proton and the neutron is much larger than that of electrons, almost all the mass is located in the nucleus. Ion: a charged particle; # protons ≠ # electrons Electrons occupy most of the volume of an ato ...
... Nucleus: the center of the atom; composed of neutrons and protons. Because the mass of the proton and the neutron is much larger than that of electrons, almost all the mass is located in the nucleus. Ion: a charged particle; # protons ≠ # electrons Electrons occupy most of the volume of an ato ...
Atomic Structure
... a) Three isotopes of sulfur are sulfur-32, sulfur33, and sulfur-34. Write the complete symbol for each isotope, including the atomic number and the mass number. b) How many neutrons, protons, and electrons are in Na+ with a mass number of 24? What is its atomic number? ...
... a) Three isotopes of sulfur are sulfur-32, sulfur33, and sulfur-34. Write the complete symbol for each isotope, including the atomic number and the mass number. b) How many neutrons, protons, and electrons are in Na+ with a mass number of 24? What is its atomic number? ...
Promethium

Promethium, originally prometheum, is a chemical element with symbol Pm and atomic number 61. All of its isotopes are radioactive; it is one of only two such elements that are followed in the periodic table by elements with stable forms, a distinction shared with technetium. Chemically, promethium is a lanthanide, which forms salts when combined with other elements. Promethium shows only one stable oxidation state of +3; however, a few +2 compounds may exist.In 1902, Bohuslav Brauner suggested there was an element with properties intermediate between those of the known elements neodymium (60) and samarium (62); this was confirmed in 1914 by Henry Moseley who, having measured the atomic numbers of all the elements then known, found there was an element with atomic number 61. In 1926, an Italian and an American group claimed to have isolated a sample of element 61; both ""discoveries"" were soon proven to be false. In 1938, during a nuclear experiment conducted at Ohio State University, a few radioactive nuclides were produced that certainly were not radioisotopes of neodymium or samarium, but there was a lack of chemical proof that element 61 was produced, and the discovery was not generally recognized. Promethium was first produced and characterized at Oak Ridge National Laboratory in 1945 by the separation and analysis of the fission products of uranium fuel irradiated in a graphite reactor. The discoverers proposed the name ""prometheum"" (the spelling was subsequently changed), derived from Prometheus, the Titan in Greek mythology who stole fire from Mount Olympus and brought it down to humans, to symbolize ""both the daring and the possible misuse of mankind's intellect"". However, a sample of the metal was made only in 1963.There are two possible sources for natural promethium: rare decays of natural europium-151 (producing promethium-147), and uranium (various isotopes). Practical applications exist only for chemical compounds of promethium-147, which are used in luminous paint, atomic batteries, and thickness measurement devices, even though promethium-145 is the most stable promethium isotope. Because natural promethium is exceedingly scarce, it is typically synthesized by bombarding uranium-235 (enriched uranium) with thermal neutrons to produce promethium-147.