Chapter 23 (Section 3) Pregnancy, Birth, and
... *a. SCIENTIFIC NOTATION is a ________________ written as a product with two FACTORS 1. The 1st FACTOR is a single digit whole number greater than ___, but less than __ 2. The 2nd FACTOR is a ____________ of 10 in exponential form [(e.g.) “105” ] *b. Steps for writing a NUMBER in a SCIENTIFIC NOTATIO ...
... *a. SCIENTIFIC NOTATION is a ________________ written as a product with two FACTORS 1. The 1st FACTOR is a single digit whole number greater than ___, but less than __ 2. The 2nd FACTOR is a ____________ of 10 in exponential form [(e.g.) “105” ] *b. Steps for writing a NUMBER in a SCIENTIFIC NOTATIO ...
Christopher Warner Title: Element Project Educational Filters: The
... - Student must research the percent weight of each isotope of their element. Limit the number of isotopes to five, and make sure to instruct the students that the percentages must sum up to 100%. -Number of protons -Number of protons = atomic number -Number of neutrons -Students must choose an isoto ...
... - Student must research the percent weight of each isotope of their element. Limit the number of isotopes to five, and make sure to instruct the students that the percentages must sum up to 100%. -Number of protons -Number of protons = atomic number -Number of neutrons -Students must choose an isoto ...
Atoms - Issaquah Connect
... • ALL atoms of the same element have the same number of protons. • All neutral atoms have no overall (net) charge, so … have the same number of electrons as protons • BUT… they can have different numbers of neutrons These are called isotopes of carbon ...
... • ALL atoms of the same element have the same number of protons. • All neutral atoms have no overall (net) charge, so … have the same number of electrons as protons • BUT… they can have different numbers of neutrons These are called isotopes of carbon ...
Explain: Determining the Subatomic Particles of Atoms
... 3. Identify the number of __________________. __________________ the __________________ to the nearest __________________. The __________________ represents the number of __________________ plus the number of __________________ in the nucleus. A.) Fluorine has an __________________ of 18.998. Round ...
... 3. Identify the number of __________________. __________________ the __________________ to the nearest __________________. The __________________ represents the number of __________________ plus the number of __________________ in the nucleus. A.) Fluorine has an __________________ of 18.998. Round ...
GEO143_lab_3_atoms_m..
... 1. Color in the Molecule Color Key with colored pencils as indicated. 2. Determine the number and type of elements in each molecule and write it down on the activity sheet. 3. Draw and color the molecule models using the colored pencils. What determines how atoms and molecules are structured? The ar ...
... 1. Color in the Molecule Color Key with colored pencils as indicated. 2. Determine the number and type of elements in each molecule and write it down on the activity sheet. 3. Draw and color the molecule models using the colored pencils. What determines how atoms and molecules are structured? The ar ...
Which of the following statements correctly describes the relative
... Neutrons are positive, electrons are neutral, protons are ...
... Neutrons are positive, electrons are neutral, protons are ...
cOO The.Parts of the Atom J
... a t o m s missing or having extra electrons. Since the number of protons an atom contains determines the a t o m , neutron n u m b e r s can c h a n g e , but the atom is still the s a m e . Let's take a closer look at carbon and see if this makes sense. Carbon is carbon b e c a u s e it has six pro ...
... a t o m s missing or having extra electrons. Since the number of protons an atom contains determines the a t o m , neutron n u m b e r s can c h a n g e , but the atom is still the s a m e . Let's take a closer look at carbon and see if this makes sense. Carbon is carbon b e c a u s e it has six pro ...
chapter 4
... but different than A and BA element Atoms of element A and B can be can be physically chemically combined mixed together as a compound ...
... but different than A and BA element Atoms of element A and B can be can be physically chemically combined mixed together as a compound ...
Key Concept Summary - Bellingham High School
... and have the same properties as electrons. A third form of radiation, that is not affected by an electric field was discovered in 1900 by Paul Villard. This radiation, called -ray, is not made up of particles; it is electromagnetic radiation of extremely high penetrating power. Properties of the th ...
... and have the same properties as electrons. A third form of radiation, that is not affected by an electric field was discovered in 1900 by Paul Villard. This radiation, called -ray, is not made up of particles; it is electromagnetic radiation of extremely high penetrating power. Properties of the th ...
Ch#4 Atoms and Elements
... Hydrogen has three isotopes. The first two protium and deuterium are stable isotopes and the third tritium is unstable, thus excluded in the calculation. The relative abundance of isotopes of a particular element is constant here on our planet Earth. Mass spectroscopy gives information of relative a ...
... Hydrogen has three isotopes. The first two protium and deuterium are stable isotopes and the third tritium is unstable, thus excluded in the calculation. The relative abundance of isotopes of a particular element is constant here on our planet Earth. Mass spectroscopy gives information of relative a ...
1.1 - cloudfront.net
... If we were to place a sample of carbon into a mass spectrometer and analyze its mass, we would find that some of the carbon atoms have a relative mass of 12, while other atoms have a relative mass of 13, and still others have a relative mass of 14. The mass spectrometer measures the percent abundanc ...
... If we were to place a sample of carbon into a mass spectrometer and analyze its mass, we would find that some of the carbon atoms have a relative mass of 12, while other atoms have a relative mass of 13, and still others have a relative mass of 14. The mass spectrometer measures the percent abundanc ...
Ch 3 Sec 3 Highlighted
... Mass Number: the total number of protons and neutrons in the nucleus of an isotope. From the periodic table the Mass Number is the atomic mass rounded to the nearest whole number. SECTION 3-3 OBJECTIVE 3 Given the identity of a nuclide, determine its number of protons, neutrons, and electrons. ...
... Mass Number: the total number of protons and neutrons in the nucleus of an isotope. From the periodic table the Mass Number is the atomic mass rounded to the nearest whole number. SECTION 3-3 OBJECTIVE 3 Given the identity of a nuclide, determine its number of protons, neutrons, and electrons. ...
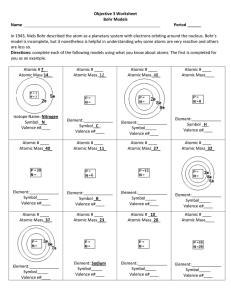
Objective 3 Worksheet Bohr Models Name Period In 1943, Niels
... Objective 3 Worksheet Bohr Models Name ...
... Objective 3 Worksheet Bohr Models Name ...
Periodic Table of Elements
... in order of increasing atomic mass. Both left vacant spaces where unknown elements should fit. So why is Mendeleev called the “father of the modern periodic table” and not Meyer, or both? ...
... in order of increasing atomic mass. Both left vacant spaces where unknown elements should fit. So why is Mendeleev called the “father of the modern periodic table” and not Meyer, or both? ...
File - Rogers` Honors Chemistry
... • The isotopes of a particular element all have the same number of protons and electrons but different numbers of neutrons. • Most of the elements consist of mixtures of isotopes. • The mass number is the total number of protons and neutrons that make up the nucleus of an ...
... • The isotopes of a particular element all have the same number of protons and electrons but different numbers of neutrons. • Most of the elements consist of mixtures of isotopes. • The mass number is the total number of protons and neutrons that make up the nucleus of an ...
Atom 3 Isotopes - Solon City Schools
... They are the same element, are chemically identical and undergo the exact same chemical reactions They have different masses (different mass number). All isotopes are used to calculate average atomic mass (this mass is usually a decimal). Most elements consist of a mixture of isotopes. ...
... They are the same element, are chemically identical and undergo the exact same chemical reactions They have different masses (different mass number). All isotopes are used to calculate average atomic mass (this mass is usually a decimal). Most elements consist of a mixture of isotopes. ...
Chapter 4 Homework 4 File
... Atoms of the same element can have different mass numbers. The nucleus of an atom has a positive charge. Atoms of isotopes of an element can have different number of protons. Atoms are mostly empty space. ...
... Atoms of the same element can have different mass numbers. The nucleus of an atom has a positive charge. Atoms of isotopes of an element can have different number of protons. Atoms are mostly empty space. ...
Atomic Theory, Mole Relationships, Percent Compositions, and
... • Metals: shiny, silvery, soft (malleable), good conductors of heat/electricity, react violently in water, all solid (except Hg). • Nonmetals: not silvery (some colored), brittle, poor conductors, some solid, liquid (Br) and gas. • Semimetals (metalloids): Have properties that cross between metals ...
... • Metals: shiny, silvery, soft (malleable), good conductors of heat/electricity, react violently in water, all solid (except Hg). • Nonmetals: not silvery (some colored), brittle, poor conductors, some solid, liquid (Br) and gas. • Semimetals (metalloids): Have properties that cross between metals ...
TEST on Atomic Structure
... TRUE or FALSE - the atomic mass increases by ONE from element to element TRUE or FALSE - the elements become more non metallic TRUE or FALSE - the ionization energy of the elements generally decreases TRUE or FALSE - the elements are arranged according to increasing atomic number TRUE or FALSE - eac ...
... TRUE or FALSE - the atomic mass increases by ONE from element to element TRUE or FALSE - the elements become more non metallic TRUE or FALSE - the ionization energy of the elements generally decreases TRUE or FALSE - the elements are arranged according to increasing atomic number TRUE or FALSE - eac ...
Chemistry
... Gain electrons – becomes negative charge = to number gained Lose electron – becomes positive charge = to number lost VII. Isotopes of an element Atoms of the same element must have the same number of protons and electrons, but they can have different numbers of neutrons Isotopes are atoms of an elem ...
... Gain electrons – becomes negative charge = to number gained Lose electron – becomes positive charge = to number lost VII. Isotopes of an element Atoms of the same element must have the same number of protons and electrons, but they can have different numbers of neutrons Isotopes are atoms of an elem ...
Student Exploration Sheet: Growing Plants
... 3. Analyze: An isotope is an alternative form of an element. Each isotope of an element has the same number of protons, but a different number of neutrons. The isotope is represented by the atomic symbol and mass number, such as He-4. Some isotopes are stable, while others are radioactive, which mea ...
... 3. Analyze: An isotope is an alternative form of an element. Each isotope of an element has the same number of protons, but a different number of neutrons. The isotope is represented by the atomic symbol and mass number, such as He-4. Some isotopes are stable, while others are radioactive, which mea ...
Name: Date:______ Period:____ Isotopes and Atomic Mass
... 'atom' was divisible. An atom is classified according to the number of protons in its nucleus: the number of protons determines the chemical element, and the number of neutrons determines the isotope of the element. Isotopes are atoms of the same element having different mass numbers due to differen ...
... 'atom' was divisible. An atom is classified according to the number of protons in its nucleus: the number of protons determines the chemical element, and the number of neutrons determines the isotope of the element. Isotopes are atoms of the same element having different mass numbers due to differen ...
2013 atoms
... Matter is not negatively charged, so atoms can’t be negatively charged either. If atoms contained extremely light, negatively charged particles, then they must also contain positively charged particles — probably with a much greater mass than electrons. ...
... Matter is not negatively charged, so atoms can’t be negatively charged either. If atoms contained extremely light, negatively charged particles, then they must also contain positively charged particles — probably with a much greater mass than electrons. ...
Promethium

Promethium, originally prometheum, is a chemical element with symbol Pm and atomic number 61. All of its isotopes are radioactive; it is one of only two such elements that are followed in the periodic table by elements with stable forms, a distinction shared with technetium. Chemically, promethium is a lanthanide, which forms salts when combined with other elements. Promethium shows only one stable oxidation state of +3; however, a few +2 compounds may exist.In 1902, Bohuslav Brauner suggested there was an element with properties intermediate between those of the known elements neodymium (60) and samarium (62); this was confirmed in 1914 by Henry Moseley who, having measured the atomic numbers of all the elements then known, found there was an element with atomic number 61. In 1926, an Italian and an American group claimed to have isolated a sample of element 61; both ""discoveries"" were soon proven to be false. In 1938, during a nuclear experiment conducted at Ohio State University, a few radioactive nuclides were produced that certainly were not radioisotopes of neodymium or samarium, but there was a lack of chemical proof that element 61 was produced, and the discovery was not generally recognized. Promethium was first produced and characterized at Oak Ridge National Laboratory in 1945 by the separation and analysis of the fission products of uranium fuel irradiated in a graphite reactor. The discoverers proposed the name ""prometheum"" (the spelling was subsequently changed), derived from Prometheus, the Titan in Greek mythology who stole fire from Mount Olympus and brought it down to humans, to symbolize ""both the daring and the possible misuse of mankind's intellect"". However, a sample of the metal was made only in 1963.There are two possible sources for natural promethium: rare decays of natural europium-151 (producing promethium-147), and uranium (various isotopes). Practical applications exist only for chemical compounds of promethium-147, which are used in luminous paint, atomic batteries, and thickness measurement devices, even though promethium-145 is the most stable promethium isotope. Because natural promethium is exceedingly scarce, it is typically synthesized by bombarding uranium-235 (enriched uranium) with thermal neutrons to produce promethium-147.