CP Chemistry Atomic Structure TEST 1. The Greek philosopher
... 3. _____ devised an oil drop experiment to determine the charge on an electron. A. Chadwick B. Moseley C. Millikan D. Thomson 4. The model of the atom that consisted of a nucleus with electrons orbiting it in energy levels like planets around the sun was proposed by A. Thomson B. Democritus C. Bohr ...
... 3. _____ devised an oil drop experiment to determine the charge on an electron. A. Chadwick B. Moseley C. Millikan D. Thomson 4. The model of the atom that consisted of a nucleus with electrons orbiting it in energy levels like planets around the sun was proposed by A. Thomson B. Democritus C. Bohr ...
Chapter 05
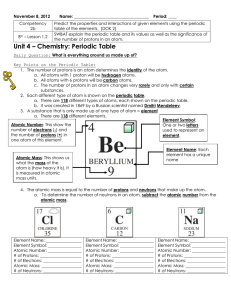
... the symbol of the element, the mass number and the atomic number. Mass number Atomic number ...
... the symbol of the element, the mass number and the atomic number. Mass number Atomic number ...
Periodic Table Notes
... • The periodic table is a chart containing information about the atoms that make up all matter. • It is called the periodic table of elements because elements are made of the same kind of atom. ...
... • The periodic table is a chart containing information about the atoms that make up all matter. • It is called the periodic table of elements because elements are made of the same kind of atom. ...
Atomic Structure-PRACTICE TEST
... TRUE or FALSE - the atomic mass increases by ONE from element to element (atomic number) TRUE or FALSE - the elements become more non metallic TRUE or FALSE - the ionization energy of the elements generally decreases TRUE or FALSE - the elements are arranged according to increasing atomic number TRU ...
... TRUE or FALSE - the atomic mass increases by ONE from element to element (atomic number) TRUE or FALSE - the elements become more non metallic TRUE or FALSE - the ionization energy of the elements generally decreases TRUE or FALSE - the elements are arranged according to increasing atomic number TRU ...
atom
... the symbol of the element, the mass number and the atomic number. Mass number Atomic number ...
... the symbol of the element, the mass number and the atomic number. Mass number Atomic number ...
Atom Internet Scavenger Hunt
... Halogens are found in Group 17 on the Periodic Table. They are non-metals and are considered salt compounds. These elements exist in all three states: liquid, solid, and gas. There are 7 electrons in the outer shell and their oxidation number is -1 and they are considered to be reactive. Elements in ...
... Halogens are found in Group 17 on the Periodic Table. They are non-metals and are considered salt compounds. These elements exist in all three states: liquid, solid, and gas. There are 7 electrons in the outer shell and their oxidation number is -1 and they are considered to be reactive. Elements in ...
atom
... the symbol of the element, the mass number and the atomic number. Mass number Atomic number ...
... the symbol of the element, the mass number and the atomic number. Mass number Atomic number ...
Intro to the Periodic Table
... a. All atoms with 1 proton will be hydrogen atoms. b. All atoms with 6 protons will be carbon atoms. c. The number of protons in an atom changes very rarely and only with certain substances. 2. Each different type of atom is shown on the periodic table. a. There are 118 different types of atoms, eac ...
... a. All atoms with 1 proton will be hydrogen atoms. b. All atoms with 6 protons will be carbon atoms. c. The number of protons in an atom changes very rarely and only with certain substances. 2. Each different type of atom is shown on the periodic table. a. There are 118 different types of atoms, eac ...
Atomic Theory PPT
... mass is calculated by adding the % of 1H mass found in nature to the % of 2H mass found in nature plus the % of 3H mass. o % 1H + % 2H + % 3H = average mass (atomic mass) o Generally the formula used is: % X + % Y + % Z… = atomic mass. An instrument called the mass spectrometer is generally used to ...
... mass is calculated by adding the % of 1H mass found in nature to the % of 2H mass found in nature plus the % of 3H mass. o % 1H + % 2H + % 3H = average mass (atomic mass) o Generally the formula used is: % X + % Y + % Z… = atomic mass. An instrument called the mass spectrometer is generally used to ...
Chemistry Topic III – The Atom
... d. Atoms of different elements can physically mix together or can chemically combine in simple whole-number ratios to form compounds. e. Chemical reactions occur when atoms are ____________________, ____________________ or ______________________. Atoms of one element, however, are never changed into ...
... d. Atoms of different elements can physically mix together or can chemically combine in simple whole-number ratios to form compounds. e. Chemical reactions occur when atoms are ____________________, ____________________ or ______________________. Atoms of one element, however, are never changed into ...
Atoms, Molecules and Ions
... always a small whole number • law states that when two elements form a series of compounds the ratios of the masses to second element to 1g of the first element can always be reduced to small whole numbers ...
... always a small whole number • law states that when two elements form a series of compounds the ratios of the masses to second element to 1g of the first element can always be reduced to small whole numbers ...
Topic 1 – Atomic structure and the periodic table
... Unlike other chemists before him, Mendeleev: o sometimes broke the ‘increasing atomic mass rule’ e.g he switched tellurium and iodine around so that they would be in the same groups as elements with similar properties (i.e by switching them, iodine was next to bromine, chlorine, fluorine…) o reali ...
... Unlike other chemists before him, Mendeleev: o sometimes broke the ‘increasing atomic mass rule’ e.g he switched tellurium and iodine around so that they would be in the same groups as elements with similar properties (i.e by switching them, iodine was next to bromine, chlorine, fluorine…) o reali ...
The ocean is a mixture.
... another and to other metals, but their properties do not fit in with those of any other family. Many transition metals combine chemically with oxygen to form compounds called oxides. They have one or two electrons in the outer level Reactivity: less reactive than alkaline-earth metals Properties: Sh ...
... another and to other metals, but their properties do not fit in with those of any other family. Many transition metals combine chemically with oxygen to form compounds called oxides. They have one or two electrons in the outer level Reactivity: less reactive than alkaline-earth metals Properties: Sh ...
Chapter 2 Atoms and Radioactivity Outline 2.1 Atoms and Their
... – For example, nitrogen-14 has seven protons and seven neutrons. – The atomic mass is the average atomic mass for all the isotopes of an element found in nature. – This number is found on the periodic table often below the element symbol. © 2014 Pearson Education, Inc. ...
... – For example, nitrogen-14 has seven protons and seven neutrons. – The atomic mass is the average atomic mass for all the isotopes of an element found in nature. – This number is found on the periodic table often below the element symbol. © 2014 Pearson Education, Inc. ...
Atomic Number
... – A unit called the atomic mass unit, or amu, is used when discussing atoms. – An amu is one-twelfth the mass of a carbon atom. – A proton and neutron each weigh 1 amu. – The mass of an electron is about 2000 times less than that of a proton or neutron. ...
... – A unit called the atomic mass unit, or amu, is used when discussing atoms. – An amu is one-twelfth the mass of a carbon atom. – A proton and neutron each weigh 1 amu. – The mass of an electron is about 2000 times less than that of a proton or neutron. ...
Intro to Nuclear Physics (Science 10 Review... Yes I know...)
... will, no doubt, recall that elements are organized by atomic number in the periodic table). A periodic table is included at the end of this section of the text. You also have one available in your CCHS planner. Z is the symbol for the atomic number. The mass number is the number of nucleons in an at ...
... will, no doubt, recall that elements are organized by atomic number in the periodic table). A periodic table is included at the end of this section of the text. You also have one available in your CCHS planner. Z is the symbol for the atomic number. The mass number is the number of nucleons in an at ...
Atomic Structure PowerPoint Presentation
... Law of Multiple Proportions o The Law of Multiple Proportions states that atoms of two or more elements may combine in different ratios to produce more than one compound ...
... Law of Multiple Proportions o The Law of Multiple Proportions states that atoms of two or more elements may combine in different ratios to produce more than one compound ...
atom`s - Hauppauge School District
... stable electron configuration of eight • Determine how many electrons are gained or lost • Write the new electron configuration Element ...
... stable electron configuration of eight • Determine how many electrons are gained or lost • Write the new electron configuration Element ...
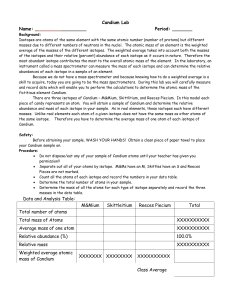
Candium Lab - OCPS TeacherPress
... masses due to different numbers of neutrons in the nuclei. The atomic mass of an element is the weighted average of the masses of the different isotopes. The weighted average takes into account both the masses of the isotopes and their relative (percent) abundance of each isotope as it occurs in nat ...
... masses due to different numbers of neutrons in the nuclei. The atomic mass of an element is the weighted average of the masses of the different isotopes. The weighted average takes into account both the masses of the isotopes and their relative (percent) abundance of each isotope as it occurs in nat ...
Chemistry Unit 2 - Finding Patterns
... The periodic table, arranged by atomic number, reveals a tendency for properties to repeat in a periodic pattern (periodicity), and can be used to predict the properties and uses of an element. These periodic trends exist for many properties of the elements including atomic radii, ionization energy, ...
... The periodic table, arranged by atomic number, reveals a tendency for properties to repeat in a periodic pattern (periodicity), and can be used to predict the properties and uses of an element. These periodic trends exist for many properties of the elements including atomic radii, ionization energy, ...
ExamView - chap 4 retake 2013.tst
... ____ 17. The atomic number indicates __________. A. the number of different isotopes of an element B. the number of atoms in 1 g of an element C. the number of neutrons in a nucleus D. the number of protons or electrons in a neutral atom E. the total number of neutrons and protons in a nucleus ____ ...
... ____ 17. The atomic number indicates __________. A. the number of different isotopes of an element B. the number of atoms in 1 g of an element C. the number of neutrons in a nucleus D. the number of protons or electrons in a neutral atom E. the total number of neutrons and protons in a nucleus ____ ...
Isotopes - Ms. Bergman`s Classes at DCIS Montbello
... How many electrons are in the isotope 23Na? Na has 11 protons, so if its neutral it has 11 electrons ...
... How many electrons are in the isotope 23Na? Na has 11 protons, so if its neutral it has 11 electrons ...
Atom
... nucleus and depends on the number of protons and neutrons. Mass number – the total number of protons and neutrons in an atom Example: Helium atom contains 2 protons and 2 neutrons, so its mass number is 4 If you know the atomic number and mass number of an atom of any element, you can determine the ...
... nucleus and depends on the number of protons and neutrons. Mass number – the total number of protons and neutrons in an atom Example: Helium atom contains 2 protons and 2 neutrons, so its mass number is 4 If you know the atomic number and mass number of an atom of any element, you can determine the ...
Promethium

Promethium, originally prometheum, is a chemical element with symbol Pm and atomic number 61. All of its isotopes are radioactive; it is one of only two such elements that are followed in the periodic table by elements with stable forms, a distinction shared with technetium. Chemically, promethium is a lanthanide, which forms salts when combined with other elements. Promethium shows only one stable oxidation state of +3; however, a few +2 compounds may exist.In 1902, Bohuslav Brauner suggested there was an element with properties intermediate between those of the known elements neodymium (60) and samarium (62); this was confirmed in 1914 by Henry Moseley who, having measured the atomic numbers of all the elements then known, found there was an element with atomic number 61. In 1926, an Italian and an American group claimed to have isolated a sample of element 61; both ""discoveries"" were soon proven to be false. In 1938, during a nuclear experiment conducted at Ohio State University, a few radioactive nuclides were produced that certainly were not radioisotopes of neodymium or samarium, but there was a lack of chemical proof that element 61 was produced, and the discovery was not generally recognized. Promethium was first produced and characterized at Oak Ridge National Laboratory in 1945 by the separation and analysis of the fission products of uranium fuel irradiated in a graphite reactor. The discoverers proposed the name ""prometheum"" (the spelling was subsequently changed), derived from Prometheus, the Titan in Greek mythology who stole fire from Mount Olympus and brought it down to humans, to symbolize ""both the daring and the possible misuse of mankind's intellect"". However, a sample of the metal was made only in 1963.There are two possible sources for natural promethium: rare decays of natural europium-151 (producing promethium-147), and uranium (various isotopes). Practical applications exist only for chemical compounds of promethium-147, which are used in luminous paint, atomic batteries, and thickness measurement devices, even though promethium-145 is the most stable promethium isotope. Because natural promethium is exceedingly scarce, it is typically synthesized by bombarding uranium-235 (enriched uranium) with thermal neutrons to produce promethium-147.