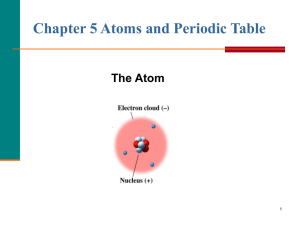
Materials Required
... -Handout on common elemental isotopes and their abundancy -Post-assessment sheet (same as pre-assessment with additional conceptual questions) Activities Gaining Attention: The term Sub-atomic particles will be written on the board as the students take their seats. As class begins a bag of candy for ...
... -Handout on common elemental isotopes and their abundancy -Post-assessment sheet (same as pre-assessment with additional conceptual questions) Activities Gaining Attention: The term Sub-atomic particles will be written on the board as the students take their seats. As class begins a bag of candy for ...
Chapter 2 Atoms and Molecules
... followed by (s) as in NaCl (s). Solids have a fixed shape because they are composed of a rigis, well define matrix locking the atoms into place. Liquids - Denoted by (l) in a chemical formula. Here there are intramolecular forces holding the individual molecules in contact with each other so there i ...
... followed by (s) as in NaCl (s). Solids have a fixed shape because they are composed of a rigis, well define matrix locking the atoms into place. Liquids - Denoted by (l) in a chemical formula. Here there are intramolecular forces holding the individual molecules in contact with each other so there i ...
Lecture 4
... A mixture is a type of matter that consists of: • Two or more substances that are physically mixed, not chemically combined • Two or more substances in different proportions • Substances that can be separated by physical methods Example: Pasta and water can be separated with a strainer. ...
... A mixture is a type of matter that consists of: • Two or more substances that are physically mixed, not chemically combined • Two or more substances in different proportions • Substances that can be separated by physical methods Example: Pasta and water can be separated with a strainer. ...
ATOMIC STRUacad test
... a. Mg b. Na c. Mn d. Fe _____9. Which element is located in period 5, group IIB? a. w b.Ag c.Mo d.Cd _____10. Which element is located in period 2, group VIA? a. S b. Se c. O ...
... a. Mg b. Na c. Mn d. Fe _____9. Which element is located in period 5, group IIB? a. w b.Ag c.Mo d.Cd _____10. Which element is located in period 2, group VIA? a. S b. Se c. O ...
Symbols of Elements - Chemistry with Mr. Patmos
... Isotopes of Magnesium In naturally occurring magnesium, there are three isotopes. Isotopes of Mg ...
... Isotopes of Magnesium In naturally occurring magnesium, there are three isotopes. Isotopes of Mg ...
notes-part-1
... describing known substances and discovering new compounds. Applied chemistry is the search for uses for chemical substances. New chemicals are being discovered or produced every day. Isotopes and Atomic Mass . Investigations conducted as long ago as the late 1700's revealed that atoms of different e ...
... describing known substances and discovering new compounds. Applied chemistry is the search for uses for chemical substances. New chemicals are being discovered or produced every day. Isotopes and Atomic Mass . Investigations conducted as long ago as the late 1700's revealed that atoms of different e ...
02_Lecture SK
... – A unit called the atomic mass unit, or amu, is used when discussing atoms. – An amu is one-twelfth the mass of a carbon atom. – A proton and neutron each weigh 1 amu. – The mass of an electron is about 2000 times less than that of a proton or neutron. ...
... – A unit called the atomic mass unit, or amu, is used when discussing atoms. – An amu is one-twelfth the mass of a carbon atom. – A proton and neutron each weigh 1 amu. – The mass of an electron is about 2000 times less than that of a proton or neutron. ...
Topic 2 Atomic Structure File
... 6. If the mass number of the atom of a given element is known, the number of neutrons in its nucleus can be calculated by subtracting the _______________________ from the _______________________. For example, if an atom of the element sodium, atomic number 11, has a mass of 23, the atom has ________ ...
... 6. If the mass number of the atom of a given element is known, the number of neutrons in its nucleus can be calculated by subtracting the _______________________ from the _______________________. For example, if an atom of the element sodium, atomic number 11, has a mass of 23, the atom has ________ ...
Chapter 4 ppt.
... If an element has an atomic number of 34 and a mass number of 78, what is the: a) number of protons b) number of neutrons c) number of electrons ...
... If an element has an atomic number of 34 and a mass number of 78, what is the: a) number of protons b) number of neutrons c) number of electrons ...
Chapter 5
... Let’s use a penny as an example (picture, in slide show, is approximately life-size). A penny, if made of pure Cu (copper) would have 2.4 x 1022 atoms. That’s 24,000,000,000,000,000,000,000 atoms, btw. Approx. 1 cm from arrow to arrow (in slide show mode) ...
... Let’s use a penny as an example (picture, in slide show, is approximately life-size). A penny, if made of pure Cu (copper) would have 2.4 x 1022 atoms. That’s 24,000,000,000,000,000,000,000 atoms, btw. Approx. 1 cm from arrow to arrow (in slide show mode) ...
Isotopes of Hydrogen
... Mass Number: the total number of protons and neutrons in the nucleus of an isotope. From the periodic table the Mass Number is the atomic mass rounded to the nearest whole number. SECTION 3-3 OBJECTIVE 3 Given the identity of a nuclide; determine its number of protons, neutrons, and electrons. Nucli ...
... Mass Number: the total number of protons and neutrons in the nucleus of an isotope. From the periodic table the Mass Number is the atomic mass rounded to the nearest whole number. SECTION 3-3 OBJECTIVE 3 Given the identity of a nuclide; determine its number of protons, neutrons, and electrons. Nucli ...
Document
... Atoms of an element that have a different number of neutrons in the nucleus are called isotopes of each other. ...
... Atoms of an element that have a different number of neutrons in the nucleus are called isotopes of each other. ...
1 - Atomic Theory - Crestwood Local Schools
... same two elements, then the ratio of the masses of those elements will always exist as a ratio of small whole numbers. (John Dalton – 1808) ...
... same two elements, then the ratio of the masses of those elements will always exist as a ratio of small whole numbers. (John Dalton – 1808) ...
Key - Seattle Central College
... – Lanthanide series: Ce-Lu, also called rare earth metals, make up <0.005% of Earth's crust – Actinide series: Th-Lr, also called transuranium elements, generally all man-made and exist for only very short periods of time before decaying to other elements Periodic Law: ...
... – Lanthanide series: Ce-Lu, also called rare earth metals, make up <0.005% of Earth's crust – Actinide series: Th-Lr, also called transuranium elements, generally all man-made and exist for only very short periods of time before decaying to other elements Periodic Law: ...
protons, neutrons, electrons
... ATOMIC NUMBER 2. From the periodic table – how is the mass of a “regular” atom determined? ...
... ATOMIC NUMBER 2. From the periodic table – how is the mass of a “regular” atom determined? ...
Vocabulary Review
... 4. _____subatomic particle d. negatively charged particle that exists in the space surrounding at atom’s nucleus 5. _____nucleus e. positively charged particle that exists in the nucleus of an atom f. tiny bits of matter that are the building blocks of an atom Set Two-------------------------------- ...
... 4. _____subatomic particle d. negatively charged particle that exists in the space surrounding at atom’s nucleus 5. _____nucleus e. positively charged particle that exists in the nucleus of an atom f. tiny bits of matter that are the building blocks of an atom Set Two-------------------------------- ...
Atomic Structure and Periodic Table PPT
... c. Atoms of different elements can mix physically or combine chemically in whole number ratios to form compounds d. Chemical reactions occur when atoms are separated, combined or rearranged however, atoms are never changed into another atom because of a chemical reaction. ...
... c. Atoms of different elements can mix physically or combine chemically in whole number ratios to form compounds d. Chemical reactions occur when atoms are separated, combined or rearranged however, atoms are never changed into another atom because of a chemical reaction. ...
Ch 4 Powerpoint
... Exercise The pesticide known as DDT paralyzes insects by binding to their nerve cells, leading to uncontrolled firing of the nerves. Before most uses of DDT were banned in the U.S., many insects had developed a resistance to it. Write out the formula for DDT. It contains 14 carbon atoms, 9 hydrogen ...
... Exercise The pesticide known as DDT paralyzes insects by binding to their nerve cells, leading to uncontrolled firing of the nerves. Before most uses of DDT were banned in the U.S., many insects had developed a resistance to it. Write out the formula for DDT. It contains 14 carbon atoms, 9 hydrogen ...
Electron Proton Neutron
... The valency of an element is the combining capacity of that element. The valency of an element is determined by the number of valence electrons present in the atom of that element. If the number of valence electrons of the atom of an element is less than or equal to four, the ...
... The valency of an element is the combining capacity of that element. The valency of an element is determined by the number of valence electrons present in the atom of that element. If the number of valence electrons of the atom of an element is less than or equal to four, the ...
Unit 3 Notes, Practice, and Review
... 19. The atomic number is unique for every element. It also tells the number of protons in that element. Every element on the periodic table has a unique number of protons. It’s like an element’s Social Security Number. 20. Atomic number is the number of protons and electrons in an atom. To get the n ...
... 19. The atomic number is unique for every element. It also tells the number of protons in that element. Every element on the periodic table has a unique number of protons. It’s like an element’s Social Security Number. 20. Atomic number is the number of protons and electrons in an atom. To get the n ...
Elements and Compounds checklist for web
... Research task: What's in a name? • Outline the contributions of Marie Curie, Albert Einstein, Glenn Seaborg and Niels Bohr to our understanding of the structure of atoms. • Identify the elements named aft ...
... Research task: What's in a name? • Outline the contributions of Marie Curie, Albert Einstein, Glenn Seaborg and Niels Bohr to our understanding of the structure of atoms. • Identify the elements named aft ...
filled in teacher version, level 1 only
... 1. matter is composed of indivisible particles Atoms Can Be Divided, but only in a nuclear reaction 2. all atoms of a particular element are identical Does Not Account for Isotopes (atoms of the same element but a different mass due to a different number of neutrons)! 3. different elements have diff ...
... 1. matter is composed of indivisible particles Atoms Can Be Divided, but only in a nuclear reaction 2. all atoms of a particular element are identical Does Not Account for Isotopes (atoms of the same element but a different mass due to a different number of neutrons)! 3. different elements have diff ...
Interactive Notebook 2 for 2011-2012
... C-l 2 and C-14 are isotopes. Since both are carbon atoms they have the same number of protons... 6. (The atomic number of carbon is 6.) Atoms of C-l 2, like any carbon atoms must have 6 protons. In order for these atoms to have a mass number of 12 they must also contain 6 neutrons. Atoms of C-14 mus ...
... C-l 2 and C-14 are isotopes. Since both are carbon atoms they have the same number of protons... 6. (The atomic number of carbon is 6.) Atoms of C-l 2, like any carbon atoms must have 6 protons. In order for these atoms to have a mass number of 12 they must also contain 6 neutrons. Atoms of C-14 mus ...
Isotopes
... all of the atoms of a given element were identical. This idea persisted for over 100 years, until James Chadwick discovered that the nuclei of most atoms contain neutrons as well as protons. (This is a good example of how a theory changes as new observations are made.) After the discovery of the neu ...
... all of the atoms of a given element were identical. This idea persisted for over 100 years, until James Chadwick discovered that the nuclei of most atoms contain neutrons as well as protons. (This is a good example of how a theory changes as new observations are made.) After the discovery of the neu ...
Promethium

Promethium, originally prometheum, is a chemical element with symbol Pm and atomic number 61. All of its isotopes are radioactive; it is one of only two such elements that are followed in the periodic table by elements with stable forms, a distinction shared with technetium. Chemically, promethium is a lanthanide, which forms salts when combined with other elements. Promethium shows only one stable oxidation state of +3; however, a few +2 compounds may exist.In 1902, Bohuslav Brauner suggested there was an element with properties intermediate between those of the known elements neodymium (60) and samarium (62); this was confirmed in 1914 by Henry Moseley who, having measured the atomic numbers of all the elements then known, found there was an element with atomic number 61. In 1926, an Italian and an American group claimed to have isolated a sample of element 61; both ""discoveries"" were soon proven to be false. In 1938, during a nuclear experiment conducted at Ohio State University, a few radioactive nuclides were produced that certainly were not radioisotopes of neodymium or samarium, but there was a lack of chemical proof that element 61 was produced, and the discovery was not generally recognized. Promethium was first produced and characterized at Oak Ridge National Laboratory in 1945 by the separation and analysis of the fission products of uranium fuel irradiated in a graphite reactor. The discoverers proposed the name ""prometheum"" (the spelling was subsequently changed), derived from Prometheus, the Titan in Greek mythology who stole fire from Mount Olympus and brought it down to humans, to symbolize ""both the daring and the possible misuse of mankind's intellect"". However, a sample of the metal was made only in 1963.There are two possible sources for natural promethium: rare decays of natural europium-151 (producing promethium-147), and uranium (various isotopes). Practical applications exist only for chemical compounds of promethium-147, which are used in luminous paint, atomic batteries, and thickness measurement devices, even though promethium-145 is the most stable promethium isotope. Because natural promethium is exceedingly scarce, it is typically synthesized by bombarding uranium-235 (enriched uranium) with thermal neutrons to produce promethium-147.