Esters - Phillips Scientific Methods
... FYI- Back to Biology: The below molecule is produced in the first step of Glycolysis in Cellular Respiration (oxidation of glucose), as ATP ADP to start the process, and the phosphate bonds to glucose (see p. 750) 5. Phosphate esters are important biological molecules. Shown below is the structure ...
... FYI- Back to Biology: The below molecule is produced in the first step of Glycolysis in Cellular Respiration (oxidation of glucose), as ATP ADP to start the process, and the phosphate bonds to glucose (see p. 750) 5. Phosphate esters are important biological molecules. Shown below is the structure ...
Workshop 5
... same reaction at a lower temperature. The Pb-C bond energy in (CH3)4Pb is 49 kcal/mol. a. Show the initiation and propagation steps for the chlorination of CH4 using (CH3)4Pb with CH4 and Cl2. Explain why lower temperatures are needed for the halogenation reaction using (CH3)4Pb as the initiator tha ...
... same reaction at a lower temperature. The Pb-C bond energy in (CH3)4Pb is 49 kcal/mol. a. Show the initiation and propagation steps for the chlorination of CH4 using (CH3)4Pb with CH4 and Cl2. Explain why lower temperatures are needed for the halogenation reaction using (CH3)4Pb as the initiator tha ...
Chapter 18 - Aldehydes and Ketones
... bonding in aldehydes or ketones, the boiling point will be lower than those of alcohols of similar molecular weights but higher than those non-polar molecules like alkanes, ethers, etc…because of the ...
... bonding in aldehydes or ketones, the boiling point will be lower than those of alcohols of similar molecular weights but higher than those non-polar molecules like alkanes, ethers, etc…because of the ...
Study Guide for Exam 2 Chapter 12
... From their structural or line-angle formulas, write names of aromatic compounds, including those with more than one substituent on the benzene ring, and those in which the benzene ring is regarded as a substituent (phenyl ) group. From their names, draw structural formulas of aromatic compounds incl ...
... From their structural or line-angle formulas, write names of aromatic compounds, including those with more than one substituent on the benzene ring, and those in which the benzene ring is regarded as a substituent (phenyl ) group. From their names, draw structural formulas of aromatic compounds incl ...
othschem.pbworks.com
... as turning litmus paper red (an excellent test for this group only) and neutralizing base. Ethers The structure of an ether is similar to water. Rather than a hydrogen on each side of the oxygen atom, there are carbon chains. The two groups may be identical (ROR)or different( R –O-R’). There is no p ...
... as turning litmus paper red (an excellent test for this group only) and neutralizing base. Ethers The structure of an ether is similar to water. Rather than a hydrogen on each side of the oxygen atom, there are carbon chains. The two groups may be identical (ROR)or different( R –O-R’). There is no p ...
Slide 1
... • In these polymers, two different functional groups are required and for each new bond between the monomer units (shown coloured below), a small molecule (often water) is produced. • Each monomer must also have two functional groups. • This can involve two different functional groups on the same mo ...
... • In these polymers, two different functional groups are required and for each new bond between the monomer units (shown coloured below), a small molecule (often water) is produced. • Each monomer must also have two functional groups. • This can involve two different functional groups on the same mo ...
Aldehydes and Ketones
... These carbonyl compounds generally have two reaction pathways – they react with strong nucleophiles (generally, strong nucleophiles have a formal negative charge) under neutral, generally anhydrous conditions, or with weak nucleophiles (those with lone pairs, but no charge) under mild acid catalysis ...
... These carbonyl compounds generally have two reaction pathways – they react with strong nucleophiles (generally, strong nucleophiles have a formal negative charge) under neutral, generally anhydrous conditions, or with weak nucleophiles (those with lone pairs, but no charge) under mild acid catalysis ...
Document
... Cyclopropanes can be readily prepared by the addition of a carbene to the double bond of an alkene. A carbene has the general structure, R2C:, in which the central carbon is surrounded by six electrons (sextet), and is thus electron deficient. The electron-deficient carbene readily adds to an electr ...
... Cyclopropanes can be readily prepared by the addition of a carbene to the double bond of an alkene. A carbene has the general structure, R2C:, in which the central carbon is surrounded by six electrons (sextet), and is thus electron deficient. The electron-deficient carbene readily adds to an electr ...
Oxidative Addition
... When the incoming ligand is 13CO, the product contains only one labeled CO, which is cis to the newly formed acetyl group. This shows that the methyl group migrates to a coordinated CO, rather than free CO attacking the Mn−Me bond. We can tell where the labeled CO is located in the product becau ...
... When the incoming ligand is 13CO, the product contains only one labeled CO, which is cis to the newly formed acetyl group. This shows that the methyl group migrates to a coordinated CO, rather than free CO attacking the Mn−Me bond. We can tell where the labeled CO is located in the product becau ...
Chapter 20: Carboxylic Acids and Nitriles
... I don’t like this description -- it is better, I believe, shown as on the next slide ...
... I don’t like this description -- it is better, I believe, shown as on the next slide ...
Table
... AlcoholsEthers+Water Condensation reaction, eliminating H2O ; Dehydration Preparation Primary alcohols aldehydes Controlled oxidation reactions Secondary alcohols ketones Controlled oxidation reactions Pathways to other groups Aldehydes primary alcohols Addition reaction with hydrogen: hydroge ...
... AlcoholsEthers+Water Condensation reaction, eliminating H2O ; Dehydration Preparation Primary alcohols aldehydes Controlled oxidation reactions Secondary alcohols ketones Controlled oxidation reactions Pathways to other groups Aldehydes primary alcohols Addition reaction with hydrogen: hydroge ...
Assignment 4 Task 1a
... have been assigned to a new case and are working as part of a team to solve the case. Working in the laboratory you will need to have a good understanding of the conventions adopted to ensure that all chemical compounds have unambiguous names. You also need to understand how a combination of element ...
... have been assigned to a new case and are working as part of a team to solve the case. Working in the laboratory you will need to have a good understanding of the conventions adopted to ensure that all chemical compounds have unambiguous names. You also need to understand how a combination of element ...
Answers
... of hydroxide is needed. Draw a full mechanism to explain. (Hint: Before the elimination takes place, another reaction type we have learned takes place more quickly.) ...
... of hydroxide is needed. Draw a full mechanism to explain. (Hint: Before the elimination takes place, another reaction type we have learned takes place more quickly.) ...
PowerPoint **
... Why alkenyl halides such as CH3CBr=ChCH3 don’t undergo substitution upon treatment with a strong base(-NH2)? Ans: ring strain. ...
... Why alkenyl halides such as CH3CBr=ChCH3 don’t undergo substitution upon treatment with a strong base(-NH2)? Ans: ring strain. ...
Topic 16 Assessed Homework - A
... By considering the optical activity of these products formed from Q and R, explain why this method would not distinguish between Q and R. ...
... By considering the optical activity of these products formed from Q and R, explain why this method would not distinguish between Q and R. ...
Chemistry - Choithram School
... Give reason for the following : i)Haloalkanes react with KCN to form alkyl cyanides as main product while AgCN forms isocyanides as the major product. ii) Haloarenes are much less reactive than haloalkanes towards nucleophilic substitution reactions. iii)Reaction of alkyl chlorides with aqueous KOH ...
... Give reason for the following : i)Haloalkanes react with KCN to form alkyl cyanides as main product while AgCN forms isocyanides as the major product. ii) Haloarenes are much less reactive than haloalkanes towards nucleophilic substitution reactions. iii)Reaction of alkyl chlorides with aqueous KOH ...
Addition of Alcohols to Form Hemiacetals and Acetals
... Amines and aldehydes or ketones react to form hemiaminals, the nitrogen analogs of hemiacetals. The hemiaminals of primary amines then lose water to form an imine (previously, Schiff base). This is the nitrogen analog of the carbonyl group. ...
... Amines and aldehydes or ketones react to form hemiaminals, the nitrogen analogs of hemiacetals. The hemiaminals of primary amines then lose water to form an imine (previously, Schiff base). This is the nitrogen analog of the carbonyl group. ...
Name - Clark College
... When electron density is shared through resonance, there is a greater extent of electron "sharing" than hyperconjugation. Since the electrons are shared by moving through like orbitals, the allylic radical is more stable. ...
... When electron density is shared through resonance, there is a greater extent of electron "sharing" than hyperconjugation. Since the electrons are shared by moving through like orbitals, the allylic radical is more stable. ...
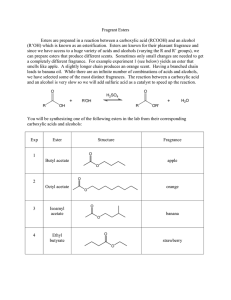
Fragrant Esters Esters are prepared in a reaction between a
... can prepare esters that produce different scents. Sometimes only small changes are needed to get a completely different fragrance. For example experiment 1 (see below) yields an ester that smells like apple. A slightly longer chain produces an orange scent. Having a branched chain leads to banana oi ...
... can prepare esters that produce different scents. Sometimes only small changes are needed to get a completely different fragrance. For example experiment 1 (see below) yields an ester that smells like apple. A slightly longer chain produces an orange scent. Having a branched chain leads to banana oi ...
Final Exam Review Sheet Chemistry 110a/1998
... cation, and anion using a resonance and molecular orbital argument. How does the allylic radical compare in stability to 3°, 2°, and 1°? How about the allylic cation, in this regard? The pKa of an allylic hydrogen is 41: how can you use this value to say that the allylic anion is more stable than th ...
... cation, and anion using a resonance and molecular orbital argument. How does the allylic radical compare in stability to 3°, 2°, and 1°? How about the allylic cation, in this regard? The pKa of an allylic hydrogen is 41: how can you use this value to say that the allylic anion is more stable than th ...
aldehyde,ketones and Haloalkanes
... Explain as to why haloarenes are much less reactive than haloalkanes towards nucleophilic substitution reactions. Which compound in each of the following pairs will react faster in SN2 reaction with – OH? Why? [3] (i) CH3Br or CH3I (ii) ...
... Explain as to why haloarenes are much less reactive than haloalkanes towards nucleophilic substitution reactions. Which compound in each of the following pairs will react faster in SN2 reaction with – OH? Why? [3] (i) CH3Br or CH3I (ii) ...
Wolff rearrangement

The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate product, which can undergo nucleophilic attack with weakly acidic nucleophiles such as water, alcohols, and amines, to generate carboxylic acid derivatives or undergo [2+2] cycloaddition reactions to form four-membered rings. The mechanism of the Wolff rearrangement has been the subject of debate since its first use. No single mechanism sufficiently describes the reaction, and there are often competing concerted and carbene-mediated pathways; for simplicity, only the textbook, concerted mechanism is shown below. The reaction was discovered by Ludwig Wolff in 1902. The Wolff rearrangement has great synthetic utility due to the accessibility of α-diazocarbonyl compounds, variety of reactions from the ketene intermediate, and stereochemical retention of the migrating group. However, the Wolff rearrangement has limitations due to the highly reactive nature of α-diazocarbonyl compounds, which can undergo a variety of competing reactions.The Wolff rearrangement can be induced via thermolysis, photolysis, or transition metal catalysis. In this last case, the reaction is sensitive to the transition metal; silver (I) oxide or other Ag(I) catalysts work well and are generally used. The Wolff rearrangement has been used in many total syntheses; the most common use is trapping the ketene intermediate with nucleophiles to form carboxylic acid derivatives. The Arndt-Eistert homologation is a specific example of this use, wherein a carboxylic acid may be elongated by a methylene unit. Another common use is in ring-contraction methods; if the α-diazo ketone is cyclic, the Wolff rearrangement results in a ring-contracted product. The Wolff rearrangement works well in generating ring-strained systems, where other reactions may fail.