CH 3502 4500
... 16. Discuss the mechanism of cleavage of ethers by HI. 17. Explain Williamson’s synthesis of ethers. 18. Discuss Norrish type-I reaction. 19. Discuss the mechanism of Wittig reaction and its uses in organic synthesis. 20. Explain Wolf-Kishner reduction with its mechanism. 21. Give any two methods o ...
... 16. Discuss the mechanism of cleavage of ethers by HI. 17. Explain Williamson’s synthesis of ethers. 18. Discuss Norrish type-I reaction. 19. Discuss the mechanism of Wittig reaction and its uses in organic synthesis. 20. Explain Wolf-Kishner reduction with its mechanism. 21. Give any two methods o ...
- professional publication
... Reaction. Mechanism and Stereochemistry of S N2 reaction, Mechanism and Stereochemistry of SN1 Reaction. Rearrangement of Carbocation, S N2 versus SN1 Reactions, Reactivity of Alkyl Halides in SN1 and SN2, Factors Affecting SN1 and SN2. ...
... Reaction. Mechanism and Stereochemistry of S N2 reaction, Mechanism and Stereochemistry of SN1 Reaction. Rearrangement of Carbocation, S N2 versus SN1 Reactions, Reactivity of Alkyl Halides in SN1 and SN2, Factors Affecting SN1 and SN2. ...
Mechanism
... The nasty smell of a dirty trash can or rotting meat is attributed mostly to amines and sulfur containing molecules. As you now know, these molecules are great at SN2 Nu’s and can do reactions in your body! Your nose is likely warning you of that! ...
... The nasty smell of a dirty trash can or rotting meat is attributed mostly to amines and sulfur containing molecules. As you now know, these molecules are great at SN2 Nu’s and can do reactions in your body! Your nose is likely warning you of that! ...
Slide 1
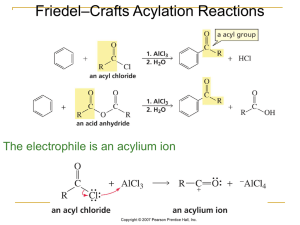
... It is not possible to obtain a good yield of an alkylbenzene containing a straight-chain group via Friedel–Crafts alkylation due to carbocation rearrangement ...
... It is not possible to obtain a good yield of an alkylbenzene containing a straight-chain group via Friedel–Crafts alkylation due to carbocation rearrangement ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 18. How are oxidizing agents chemoselective? Explain with suitable examples. 19. Explain the mechanism of Aldol condensation and Wittig reaction. 20. Discuss the mechanism of Baeyer Villiger reaction with suitable example. 21. Discuss on the electroorganic synthesis by reduction with suitable exampl ...
... 18. How are oxidizing agents chemoselective? Explain with suitable examples. 19. Explain the mechanism of Aldol condensation and Wittig reaction. 20. Discuss the mechanism of Baeyer Villiger reaction with suitable example. 21. Discuss on the electroorganic synthesis by reduction with suitable exampl ...
Chapter Nine: Alcohols, Ethers and Epoxides
... iii. Reduction with hydride reducing agent LiAlH4 : 12.6 ...
... iii. Reduction with hydride reducing agent LiAlH4 : 12.6 ...
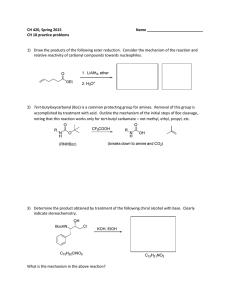
CH 420, Spring 2015 Name ___________________________ CH 18 practice problems
... 1) Draw the products of the following ester reduction. Consider the mechanism of the reaction and relative reactivity of carbonyl compounds towards nucleophiles. ...
... 1) Draw the products of the following ester reduction. Consider the mechanism of the reaction and relative reactivity of carbonyl compounds towards nucleophiles. ...
suman_organic
... Test For functional group(Amines, Amides, Substituted Amides, Nitro, Cyanide, Isocyanide,Imine, Nitroso) Separation of mixtures ...
... Test For functional group(Amines, Amides, Substituted Amides, Nitro, Cyanide, Isocyanide,Imine, Nitroso) Separation of mixtures ...
Fundamentals Of Organic Chemistry
... Reactions involving the change in the carbon skeleton through the rearrangement of the carbonium intermediate by alkyl and hydride shift collectively known as Wagner–Meerwein rearrangement. When neopentyl bromide is hydrolysed under S N1 (due to bulky alkyl group) condition it is found that instead ...
... Reactions involving the change in the carbon skeleton through the rearrangement of the carbonium intermediate by alkyl and hydride shift collectively known as Wagner–Meerwein rearrangement. When neopentyl bromide is hydrolysed under S N1 (due to bulky alkyl group) condition it is found that instead ...
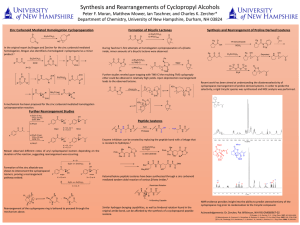
Here is the Original File - University of New Hampshire
... Further studies reveled upon trapping with TMS-Cl the resulting TMS cycloproply ether could be obtained in relatively high yields. Upon deprotection rearrangement leads to the observed lactone. ...
... Further studies reveled upon trapping with TMS-Cl the resulting TMS cycloproply ether could be obtained in relatively high yields. Upon deprotection rearrangement leads to the observed lactone. ...
Wolff rearrangement

The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate product, which can undergo nucleophilic attack with weakly acidic nucleophiles such as water, alcohols, and amines, to generate carboxylic acid derivatives or undergo [2+2] cycloaddition reactions to form four-membered rings. The mechanism of the Wolff rearrangement has been the subject of debate since its first use. No single mechanism sufficiently describes the reaction, and there are often competing concerted and carbene-mediated pathways; for simplicity, only the textbook, concerted mechanism is shown below. The reaction was discovered by Ludwig Wolff in 1902. The Wolff rearrangement has great synthetic utility due to the accessibility of α-diazocarbonyl compounds, variety of reactions from the ketene intermediate, and stereochemical retention of the migrating group. However, the Wolff rearrangement has limitations due to the highly reactive nature of α-diazocarbonyl compounds, which can undergo a variety of competing reactions.The Wolff rearrangement can be induced via thermolysis, photolysis, or transition metal catalysis. In this last case, the reaction is sensitive to the transition metal; silver (I) oxide or other Ag(I) catalysts work well and are generally used. The Wolff rearrangement has been used in many total syntheses; the most common use is trapping the ketene intermediate with nucleophiles to form carboxylic acid derivatives. The Arndt-Eistert homologation is a specific example of this use, wherein a carboxylic acid may be elongated by a methylene unit. Another common use is in ring-contraction methods; if the α-diazo ketone is cyclic, the Wolff rearrangement results in a ring-contracted product. The Wolff rearrangement works well in generating ring-strained systems, where other reactions may fail.