DISTINGUISH TESTS
... In a molecule if Carbon atom is surrounded by four different groups called asymmetric carbon or chiral carbon which is must for optical active compound. 1:1 ratio of dextro and leavo mixture is known as racemic mixture. The process of conversion of enantiomer into a racemic mixture is known as ...
... In a molecule if Carbon atom is surrounded by four different groups called asymmetric carbon or chiral carbon which is must for optical active compound. 1:1 ratio of dextro and leavo mixture is known as racemic mixture. The process of conversion of enantiomer into a racemic mixture is known as ...
1 -
... The position of the triple bond takes the lowest possible number. If both double and triple bonds are present, the ending becomes –enyne and numbering is such as to give the lowest possible numbers to the double and triple bonds, irrespective of whether –ene or –yne has the lower number. When ...
... The position of the triple bond takes the lowest possible number. If both double and triple bonds are present, the ending becomes –enyne and numbering is such as to give the lowest possible numbers to the double and triple bonds, irrespective of whether –ene or –yne has the lower number. When ...
Acidity of Alcohols
... • Yeast is killed by ethanol concentrations in excess of about 15%, and that limits the purity of the ethanol that can be produced. The ethanol is separated from the mixture by fractional distillation to give 96% pure ethanol. • For theoretical reasons, it is impossible to remove the last 4% of wate ...
... • Yeast is killed by ethanol concentrations in excess of about 15%, and that limits the purity of the ethanol that can be produced. The ethanol is separated from the mixture by fractional distillation to give 96% pure ethanol. • For theoretical reasons, it is impossible to remove the last 4% of wate ...
Dehydration of ROH
... Step 3: Proton transfer from a carbon adjacent to the positively charged carbon to water. The sigma electrons of the C-H bond become the pi electrons of the carbon-carbon double bond. H O H ...
... Step 3: Proton transfer from a carbon adjacent to the positively charged carbon to water. The sigma electrons of the C-H bond become the pi electrons of the carbon-carbon double bond. H O H ...
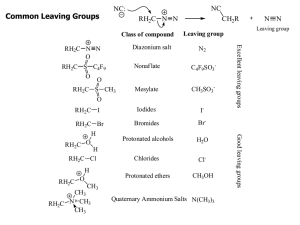
Common Leaving Groups
... •To favour E1 over SN1 for alcohols, use an acid with a non-nucleophilic conjugate base (H2SO4, H3PO4). To favour SN1 over E1, use a good nucleophile. •To favour E2 over SN2, use a strong, bulky non-nucleophilic base. To favour SN2 over E2, use good nucleophiles that are relatively weak bases. •It i ...
... •To favour E1 over SN1 for alcohols, use an acid with a non-nucleophilic conjugate base (H2SO4, H3PO4). To favour SN1 over E1, use a good nucleophile. •To favour E2 over SN2, use a strong, bulky non-nucleophilic base. To favour SN2 over E2, use good nucleophiles that are relatively weak bases. •It i ...
Organic synthesis and methodology related to the malaria drug artemisinin
... Access to artemisinin through isolation, bio-engineering, and chemical synthesis will be described. Our attempts to access the artemisinin family of anti-malarials through the total synthesis of dihydro-epi-deoxyarteannuin B and dihydroartemisinic acid will be discussed fully. Key features of the sy ...
... Access to artemisinin through isolation, bio-engineering, and chemical synthesis will be described. Our attempts to access the artemisinin family of anti-malarials through the total synthesis of dihydro-epi-deoxyarteannuin B and dihydroartemisinic acid will be discussed fully. Key features of the sy ...
M.Sc. II - Punjabi University
... (b) Ligand replacement reaction. Labile and inert complexes, crystal field stabilization, general aspects, dissociation or displacement, classification of mechanism, water exchange rates, formation of complexes from aqueous ions, equation and base hydrolysis, attack on ligands. (c) Reactions of Squa ...
... (b) Ligand replacement reaction. Labile and inert complexes, crystal field stabilization, general aspects, dissociation or displacement, classification of mechanism, water exchange rates, formation of complexes from aqueous ions, equation and base hydrolysis, attack on ligands. (c) Reactions of Squa ...
Aromatic Substitution Reactions
... ost of the reactions discussed in this chapter involve the attack of an electrophile on an aromatic compound. Although the initial step of the mechanism resembles that of the electrophilic addition reactions of carbon–carbon double bonds discussed in Chapter 11, the final product here results from s ...
... ost of the reactions discussed in this chapter involve the attack of an electrophile on an aromatic compound. Although the initial step of the mechanism resembles that of the electrophilic addition reactions of carbon–carbon double bonds discussed in Chapter 11, the final product here results from s ...
Chapter-16A
... Acid chlorides are most often prepared by treating a carboxylic acid with thionyl chloride O OH + SOCl 2 Butanoic Thionyl acid chloride ...
... Acid chlorides are most often prepared by treating a carboxylic acid with thionyl chloride O OH + SOCl 2 Butanoic Thionyl acid chloride ...
8fd26191dcc2fe1
... There are two possible ionic mechanisms for nucleophilic substitution, SN1 and SN2. S – substitution; N – nucleophilic; 1 – unimolecular (the rate determining, r.d.s., step entails one molecule); 2 – bimolecular (the rate determining step entails two species). ...
... There are two possible ionic mechanisms for nucleophilic substitution, SN1 and SN2. S – substitution; N – nucleophilic; 1 – unimolecular (the rate determining, r.d.s., step entails one molecule); 2 – bimolecular (the rate determining step entails two species). ...
Lecture - Ch 18
... • Ethers with tertiary, benzylic, or allylic groups cleave by either an SN1 or E1 mechanism – Intermediates are stable carbocations ...
... • Ethers with tertiary, benzylic, or allylic groups cleave by either an SN1 or E1 mechanism – Intermediates are stable carbocations ...
Lecture - Ch 19
... of Aldehydes and Ketones • Nucleophilic additions to aldehydes and ketones have two general variations – Product is a direct result of the tetrahedral intermediate being protonated by water or acid – Carbonyl oxygen atom is protonated and eliminated as HO- or H2O to give a product with a C=Nu double ...
... of Aldehydes and Ketones • Nucleophilic additions to aldehydes and ketones have two general variations – Product is a direct result of the tetrahedral intermediate being protonated by water or acid – Carbonyl oxygen atom is protonated and eliminated as HO- or H2O to give a product with a C=Nu double ...
Chemistry 2100 - Bonham Chemistry
... The functional group of an amide is a carbonyl group bonded to a nitrogen atom. – To name an amide, drop the suffix -oic acid from the IUPAC name of the parent acid, or -ic acid from its common name, and add -amide. – If the amide nitrogen is also bonded to an alkyl or aryl group, name the group and ...
... The functional group of an amide is a carbonyl group bonded to a nitrogen atom. – To name an amide, drop the suffix -oic acid from the IUPAC name of the parent acid, or -ic acid from its common name, and add -amide. – If the amide nitrogen is also bonded to an alkyl or aryl group, name the group and ...
74 CHAPTER-IV "LEAD (IV) ACETATE OXIDATIONS"
... saturated hydrocarbons by lead (IV) acetate in presence of short chain alcohol is considered to proceed via alkoxy radicals as transient intermediates, which abstract a hydrogen atom from cyclohexane. 15 In the oxidation of mono and disubstituted acyclic olefins with lead (IV) acetate, three competi ...
... saturated hydrocarbons by lead (IV) acetate in presence of short chain alcohol is considered to proceed via alkoxy radicals as transient intermediates, which abstract a hydrogen atom from cyclohexane. 15 In the oxidation of mono and disubstituted acyclic olefins with lead (IV) acetate, three competi ...
Mock Exam One
... a.) The bond angle around the oxygen of an alcohol is < 109.5°. b.) Alcohols contain a polar covalent bond that an alkane does not have. c.) The strongest intermolecular force present in an alcohol is hydrogen bonding while the strongest intermolecular force present in alkanes is London dispersion. ...
... a.) The bond angle around the oxygen of an alcohol is < 109.5°. b.) Alcohols contain a polar covalent bond that an alkane does not have. c.) The strongest intermolecular force present in an alcohol is hydrogen bonding while the strongest intermolecular force present in alkanes is London dispersion. ...
unit 12 aldehydes, ketones and carboxylic acids
... C6H4COOH is much stronger acid than CH3-C6H4COOH. Q.14 Explain why O-hydroxy benzaldehyde is a liquid at room temperature while p- hydroxy benzaldehyde is a high melting solid. Ans Due to intramolecular H-bonding in O-hydroxy benzaldehyde exists as discrete molecule whereas due to intermolecular H-b ...
... C6H4COOH is much stronger acid than CH3-C6H4COOH. Q.14 Explain why O-hydroxy benzaldehyde is a liquid at room temperature while p- hydroxy benzaldehyde is a high melting solid. Ans Due to intramolecular H-bonding in O-hydroxy benzaldehyde exists as discrete molecule whereas due to intermolecular H-b ...
Review
... mechanisms for benzylic only, but they look similar for allylic. None of the resonance forms are shown for the benzylic cation but they are responsible for its stability. Nu ...
... mechanisms for benzylic only, but they look similar for allylic. None of the resonance forms are shown for the benzylic cation but they are responsible for its stability. Nu ...
Anionic rearrangement of 2-benzyloxypyridine derivatives and a synthetic approach to aldingenin B
... Synthesis of alkynes by fragmentation is an on-going interest of the Dudley lab. One current goal is to apply our methodology in conjunction with an innovative oxidative alkyne ketalization to achieve a short and efficient synthesis of aldingenin B. The specific goal for this dissertation was to pre ...
... Synthesis of alkynes by fragmentation is an on-going interest of the Dudley lab. One current goal is to apply our methodology in conjunction with an innovative oxidative alkyne ketalization to achieve a short and efficient synthesis of aldingenin B. The specific goal for this dissertation was to pre ...
Nucleophilic Additions to Carbonyl Group
... group itself. Thus, even a nucleophile that is not very reactive with a carbonyl reacts readily with the conjugate acid. Of the seven functional groups containing a carbonyl group, only aldehydes and ketones commonly undergo nucleophilic addition. The remaining five carbonyl functional groups underg ...
... group itself. Thus, even a nucleophile that is not very reactive with a carbonyl reacts readily with the conjugate acid. Of the seven functional groups containing a carbonyl group, only aldehydes and ketones commonly undergo nucleophilic addition. The remaining five carbonyl functional groups underg ...
Grignard-syn-12-ques
... ethylmagnesium bromide (CH3CH2MgBr) with butanal (CH3CH2CH2CH=O) followed by dilute ...
... ethylmagnesium bromide (CH3CH2MgBr) with butanal (CH3CH2CH2CH=O) followed by dilute ...
Forward
... reactions that you already know about and are summarized in Table 17.1. To the synthetic chemist, the most important of the reactions in Table 17.1 are the last two: the oxidation of primary alcohols to aldehydes and secondary alcohols to ketones. Indeed, when combined with reactions that yield alco ...
... reactions that you already know about and are summarized in Table 17.1. To the synthetic chemist, the most important of the reactions in Table 17.1 are the last two: the oxidation of primary alcohols to aldehydes and secondary alcohols to ketones. Indeed, when combined with reactions that yield alco ...
Alcohols and Phenols
... not), forming soluble salts that are soluble in dilute aqueous • A phenolic component can be separated from an organic solution by extraction into basic aqueous solution and is isolated after acid is added to the solution ...
... not), forming soluble salts that are soluble in dilute aqueous • A phenolic component can be separated from an organic solution by extraction into basic aqueous solution and is isolated after acid is added to the solution ...
Alcohols and Phenols
... substituents come from the Grignard reagent Grignard reagents do not add to carboxylic acids – they undergo an acid-base reaction, generating the hydrocarbon of the Grignard reagent ...
... substituents come from the Grignard reagent Grignard reagents do not add to carboxylic acids – they undergo an acid-base reaction, generating the hydrocarbon of the Grignard reagent ...
Alcohols and Phenols - faculty at Chemeketa
... substituents carbon come from the Grignard reagent Grignard reagents do not add to carboxylic acids – they undergo an acid-base reaction, generating the hydrocarbon of the Grignard reagent ...
... substituents carbon come from the Grignard reagent Grignard reagents do not add to carboxylic acids – they undergo an acid-base reaction, generating the hydrocarbon of the Grignard reagent ...
Wolff rearrangement

The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate product, which can undergo nucleophilic attack with weakly acidic nucleophiles such as water, alcohols, and amines, to generate carboxylic acid derivatives or undergo [2+2] cycloaddition reactions to form four-membered rings. The mechanism of the Wolff rearrangement has been the subject of debate since its first use. No single mechanism sufficiently describes the reaction, and there are often competing concerted and carbene-mediated pathways; for simplicity, only the textbook, concerted mechanism is shown below. The reaction was discovered by Ludwig Wolff in 1902. The Wolff rearrangement has great synthetic utility due to the accessibility of α-diazocarbonyl compounds, variety of reactions from the ketene intermediate, and stereochemical retention of the migrating group. However, the Wolff rearrangement has limitations due to the highly reactive nature of α-diazocarbonyl compounds, which can undergo a variety of competing reactions.The Wolff rearrangement can be induced via thermolysis, photolysis, or transition metal catalysis. In this last case, the reaction is sensitive to the transition metal; silver (I) oxide or other Ag(I) catalysts work well and are generally used. The Wolff rearrangement has been used in many total syntheses; the most common use is trapping the ketene intermediate with nucleophiles to form carboxylic acid derivatives. The Arndt-Eistert homologation is a specific example of this use, wherein a carboxylic acid may be elongated by a methylene unit. Another common use is in ring-contraction methods; if the α-diazo ketone is cyclic, the Wolff rearrangement results in a ring-contracted product. The Wolff rearrangement works well in generating ring-strained systems, where other reactions may fail.