Organic Chemistry - Rutgers University, Newark
... Conversion of an alcohol to a sulfonate ester converts HOH, a very poor leaving group, into a sulfonic ester, a very good leaving group ...
... Conversion of an alcohol to a sulfonate ester converts HOH, a very poor leaving group, into a sulfonic ester, a very good leaving group ...
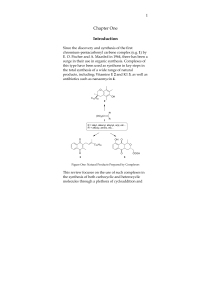
Investigating the Dearomative Rearrangement of Biaryl Phosphine- Ligated Pd(II) Complexes
... connectivity that allows for the loss of HBr and rearomatization to occur. Taken together, these reports suggest that the lower ring of biaryl phosphine ligands may not be innocent in the reactivity and decomposition pathways of catalytic intermediates. Reactions wherein a transition metal-bound are ...
... connectivity that allows for the loss of HBr and rearomatization to occur. Taken together, these reports suggest that the lower ring of biaryl phosphine ligands may not be innocent in the reactivity and decomposition pathways of catalytic intermediates. Reactions wherein a transition metal-bound are ...
Lecture 10 Carbon-Nitrogen Bonds Formation I
... Cl T.-L. Ho, R.-J. Chein, J. Org. Chem. 2004, 69, 591. ...
... Cl T.-L. Ho, R.-J. Chein, J. Org. Chem. 2004, 69, 591. ...
Learning Guide for Chapter 16
... source of H will depend on the Nu What product is formed when each of the following nucleophiles is reacted with ethylene oxide? What is the proton source in each case? O ...
... source of H will depend on the Nu What product is formed when each of the following nucleophiles is reacted with ethylene oxide? What is the proton source in each case? O ...
98 pts
... • (T) All E1 reactions involve formation of carbocations; • (T) More stable carbocations are generated faster; • (T) Carbocations are electrophiles; • (T) Carbocations are electron deficient; • (T) Free radicals are electron deficient; • (T) Alcohols are Brønsted bases; • (F) The rate-determining st ...
... • (T) All E1 reactions involve formation of carbocations; • (T) More stable carbocations are generated faster; • (T) Carbocations are electrophiles; • (T) Carbocations are electron deficient; • (T) Free radicals are electron deficient; • (T) Alcohols are Brønsted bases; • (F) The rate-determining st ...
Ester-containing polyols having halogen and phosphorus atoms
... the polyesters. Thus, polyester amides may be obtained 30 are generally prepared by heating the reactants at tem peratures between 25° C. and 150° C. preferably between ...
... the polyesters. Thus, polyester amides may be obtained 30 are generally prepared by heating the reactants at tem peratures between 25° C. and 150° C. preferably between ...
CHM 235 Course Outline and Homework in McMurry (6th ed.)
... Polar reactions (electrophile, nucleophile) Curved arrow formalism (“arrow-pushing”) to show reaction mechanisms Thermodynamics (Go = Ho - TSo) (exergonic, endergonic, exothermic, endothermic) Bond dissociation energies (Ho = energy used to break bonds–energy gained by making bonds) En ...
... Polar reactions (electrophile, nucleophile) Curved arrow formalism (“arrow-pushing”) to show reaction mechanisms Thermodynamics (Go = Ho - TSo) (exergonic, endergonic, exothermic, endothermic) Bond dissociation energies (Ho = energy used to break bonds–energy gained by making bonds) En ...
Chapter 13. Alcohols, Diols, and Ethers
... In general, difficult to cleave ether C-O bonds (for exceptions, see VII). Can be cleaved by heating with HI (more common) or HBr. Need a strong Brönsted or Lewis acid and a strong nucleophile. Usually, methyl ether C-O and benzyl ether C-O are those that can be cleaved. Modern methods for cleaving ...
... In general, difficult to cleave ether C-O bonds (for exceptions, see VII). Can be cleaved by heating with HI (more common) or HBr. Need a strong Brönsted or Lewis acid and a strong nucleophile. Usually, methyl ether C-O and benzyl ether C-O are those that can be cleaved. Modern methods for cleaving ...
Functional Derivatives of Carboxylic Acids
... • Hydrolysis in aqueous acid is the reverse of Fischer esterification – the role of the acid catalyst is to protonate the carbonyl oxygen and increase its electrophilic character toward attack by water (a weak nucleophile) to form a tetrahedral carbonyl addition intermediate – collapse of this inter ...
... • Hydrolysis in aqueous acid is the reverse of Fischer esterification – the role of the acid catalyst is to protonate the carbonyl oxygen and increase its electrophilic character toward attack by water (a weak nucleophile) to form a tetrahedral carbonyl addition intermediate – collapse of this inter ...
Organometallics II
... formula C11H22O, are formed in the reaction of methyl lithium with 3-(R)-tertbutylcyclohexanone. These two alcohols are ...
... formula C11H22O, are formed in the reaction of methyl lithium with 3-(R)-tertbutylcyclohexanone. These two alcohols are ...
Unit 4 Chemical Kinetics and Chemical Equilibrium
... NaI (E2 mechanism) Zn/HOAc (redox reaction) ...
... NaI (E2 mechanism) Zn/HOAc (redox reaction) ...
Latest Publication (still not complete)
... observed reaction pathways and hence is crucial to the total understanding of the chemistry of chromium-pentacarbonyl carbene complexes. Fischer carbene complexes exhibit two characteristic features that are important in understanding their respective chemistry. The first of these features is the fa ...
... observed reaction pathways and hence is crucial to the total understanding of the chemistry of chromium-pentacarbonyl carbene complexes. Fischer carbene complexes exhibit two characteristic features that are important in understanding their respective chemistry. The first of these features is the fa ...
Chemistry 162 Workbook 10.6
... experience with testing environments. Once the student has completed an exam, the tutor may choose to grade the exam during a normal session while the student works on other materials ...
... experience with testing environments. Once the student has completed an exam, the tutor may choose to grade the exam during a normal session while the student works on other materials ...
Chapter Seventeen
... Unsubstituted amides can form 3 strong hydrogen bonds to other amide molecules. They are higher melting and higher boiling than the acids from which they are derived. Except for the simplest amide (formamide, a liquid), the low molecular-weight unsubstituted amides are solids that are soluble in bot ...
... Unsubstituted amides can form 3 strong hydrogen bonds to other amide molecules. They are higher melting and higher boiling than the acids from which they are derived. Except for the simplest amide (formamide, a liquid), the low molecular-weight unsubstituted amides are solids that are soluble in bot ...
Chapter 16
... The alkoxide will reform a carbonyl (strong bond) with the good leaving group present ...
... The alkoxide will reform a carbonyl (strong bond) with the good leaving group present ...
Metal-catalysed approaches to amide bond formation
... 3. Amides from esters As the preparation of amides from carboxylic acids is difficult to achieve in a catalytic manner, their derivatives, particularly esters, have been explored as an alternative in catalytic amide forming reactions. In 2003, a simple procedure was published by Ranu and Dutta, using ...
... 3. Amides from esters As the preparation of amides from carboxylic acids is difficult to achieve in a catalytic manner, their derivatives, particularly esters, have been explored as an alternative in catalytic amide forming reactions. In 2003, a simple procedure was published by Ranu and Dutta, using ...
A Mild and Convenient Conversion of Ketones to the Corresponding
... to incorporate deuterium regiospecifically by using deuterium oxide as the source of deuterium.’O The purpose of this study was to investigate reducing agents which are as versatile as catecholborane but which can be more readily prepared. We report that bis(benzoyloxy)borane, I,l1J2effectively redu ...
... to incorporate deuterium regiospecifically by using deuterium oxide as the source of deuterium.’O The purpose of this study was to investigate reducing agents which are as versatile as catecholborane but which can be more readily prepared. We report that bis(benzoyloxy)borane, I,l1J2effectively redu ...
Preface (PDF, 24 Pages, 5.7 MB)
... Credits and acknowledgments borrowed from other sources and reproduced, with permission, in this textbook appear on the appropriate page within the text or on p. P-1. Copyright © 2016, 2010, 2006 Pearson Education, Inc. All rights reserved. Manufactured in the United States of America. This publica ...
... Credits and acknowledgments borrowed from other sources and reproduced, with permission, in this textbook appear on the appropriate page within the text or on p. P-1. Copyright © 2016, 2010, 2006 Pearson Education, Inc. All rights reserved. Manufactured in the United States of America. This publica ...
Working with Hazardous Chemicals
... hydrochloride with phosgene;16 however, the yield is only 36%, and hydrogen chloride must be introduced to increase the yield to 92%. The present procedure effects this reaction without additional hydrogen chloride and avoids the hazards of handling phosgene. This procedure has been successful in th ...
... hydrochloride with phosgene;16 however, the yield is only 36%, and hydrogen chloride must be introduced to increase the yield to 92%. The present procedure effects this reaction without additional hydrogen chloride and avoids the hazards of handling phosgene. This procedure has been successful in th ...
Wolff rearrangement

The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate product, which can undergo nucleophilic attack with weakly acidic nucleophiles such as water, alcohols, and amines, to generate carboxylic acid derivatives or undergo [2+2] cycloaddition reactions to form four-membered rings. The mechanism of the Wolff rearrangement has been the subject of debate since its first use. No single mechanism sufficiently describes the reaction, and there are often competing concerted and carbene-mediated pathways; for simplicity, only the textbook, concerted mechanism is shown below. The reaction was discovered by Ludwig Wolff in 1902. The Wolff rearrangement has great synthetic utility due to the accessibility of α-diazocarbonyl compounds, variety of reactions from the ketene intermediate, and stereochemical retention of the migrating group. However, the Wolff rearrangement has limitations due to the highly reactive nature of α-diazocarbonyl compounds, which can undergo a variety of competing reactions.The Wolff rearrangement can be induced via thermolysis, photolysis, or transition metal catalysis. In this last case, the reaction is sensitive to the transition metal; silver (I) oxide or other Ag(I) catalysts work well and are generally used. The Wolff rearrangement has been used in many total syntheses; the most common use is trapping the ketene intermediate with nucleophiles to form carboxylic acid derivatives. The Arndt-Eistert homologation is a specific example of this use, wherein a carboxylic acid may be elongated by a methylene unit. Another common use is in ring-contraction methods; if the α-diazo ketone is cyclic, the Wolff rearrangement results in a ring-contracted product. The Wolff rearrangement works well in generating ring-strained systems, where other reactions may fail.