full size
... aldehydes are less volatile (higher boiling) than alkanes or ethers but are more volatile than alcohols or carboxylic acids. They are slightly less soluble in water than the alcohols of similar molecular weight. δ− ¾Simple aldehydes have very O pungent and irritating odors and δ+ C are toxic. Aldehy ...
... aldehydes are less volatile (higher boiling) than alkanes or ethers but are more volatile than alcohols or carboxylic acids. They are slightly less soluble in water than the alcohols of similar molecular weight. δ− ¾Simple aldehydes have very O pungent and irritating odors and δ+ C are toxic. Aldehy ...
Regiospecificity according to Markovnikov
... • Enols rearrange to the isomeric ketone by the rapid transfer of a proton from the hydroxyl to the alkene carbon • The keto form is usually so stable compared to the enol that only the keto form can be observed ...
... • Enols rearrange to the isomeric ketone by the rapid transfer of a proton from the hydroxyl to the alkene carbon • The keto form is usually so stable compared to the enol that only the keto form can be observed ...
Learning Guide for Chapter 22 - Carboxylic Acids
... (nitriles) can also be made from carboxylic acids, as we shall now see. Acid chlorides are the most reactive of the carboxylic acid derivatives. They are made from carboxylic acids by reacting them with thionyl chloride, SOCl2, or oxalyl chloride. O ...
... (nitriles) can also be made from carboxylic acids, as we shall now see. Acid chlorides are the most reactive of the carboxylic acid derivatives. They are made from carboxylic acids by reacting them with thionyl chloride, SOCl2, or oxalyl chloride. O ...
Chem 350 Jasperse Ch. 6 Summary of Reaction Types, Ch. 4
... Stability/Reactivity/Selectivity Principles 1. Reactant Stability/Reactivity: The more stable the reactant, the less reactive it will be. In terms of rates, this means that the more stable the reactant, the slower it will react. (The concept here is that the more stable the reactant, the more conten ...
... Stability/Reactivity/Selectivity Principles 1. Reactant Stability/Reactivity: The more stable the reactant, the less reactive it will be. In terms of rates, this means that the more stable the reactant, the slower it will react. (The concept here is that the more stable the reactant, the more conten ...
Aromatic electrophilic substitution
... substituent groups because the individual effects are mutually supporting of each other. 3. In cases were there is a conflict in the directing effects of the substituent groups it can more difficult to predict what products will be produced. When dealing with multiple substituents activating groups ...
... substituent groups because the individual effects are mutually supporting of each other. 3. In cases were there is a conflict in the directing effects of the substituent groups it can more difficult to predict what products will be produced. When dealing with multiple substituents activating groups ...
REASONING QUESTIONS IN ORGANIC CHEMISTRY
... Ans. Since tertiary carbocation is more stable and reaction is following carbocation mechanism it gives t- Butyl iodide. 23. The commercial ethanol is mixed with copper sulphate & pyridine. Explain. Ans. Commercial ethanol is mixed with CuSo4 & pyridine to make it unfit for drinking. It is known as ...
... Ans. Since tertiary carbocation is more stable and reaction is following carbocation mechanism it gives t- Butyl iodide. 23. The commercial ethanol is mixed with copper sulphate & pyridine. Explain. Ans. Commercial ethanol is mixed with CuSo4 & pyridine to make it unfit for drinking. It is known as ...
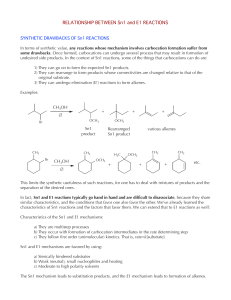
RELATIONSHIP BETWEEN Sn1 and E1 REACTIONS
... This limits the synthetic usefulness of such reactions, for one has to deal with mixtures of products and the separation of the desired ones. In fact, Sn1 and E1 reactions typically go hand in hand and are difficult to disassociate, because they share similar characteristics, and the conditions that ...
... This limits the synthetic usefulness of such reactions, for one has to deal with mixtures of products and the separation of the desired ones. In fact, Sn1 and E1 reactions typically go hand in hand and are difficult to disassociate, because they share similar characteristics, and the conditions that ...
Montmorillonite: An efficient, heterogeneous and
... MMT can form many nanocompsites with different organic compounds. Therefore, it expresses a significant capability to be used as a drug carrier for pharmaceutical purposes. Montmorillonite clay’s are layered silicates and are among the numerous inorganic supports for reagents used in organic synthes ...
... MMT can form many nanocompsites with different organic compounds. Therefore, it expresses a significant capability to be used as a drug carrier for pharmaceutical purposes. Montmorillonite clay’s are layered silicates and are among the numerous inorganic supports for reagents used in organic synthes ...
The 9-Phenyl-9-fluorenyl Group for Nitrogen Protection in
... The use of Davis’ reagent as the hydroxylating agent gives a 1:1 mixture of diastereomers under all conditions examined. N-Pf-Aspartate tert-butyl ester and free acid react with poor selectivity. Enolates generated with LiHMDS do not react at all with MoOPH [25]. The selectivity has been explained b ...
... The use of Davis’ reagent as the hydroxylating agent gives a 1:1 mixture of diastereomers under all conditions examined. N-Pf-Aspartate tert-butyl ester and free acid react with poor selectivity. Enolates generated with LiHMDS do not react at all with MoOPH [25]. The selectivity has been explained b ...
Exam 3 - Napa Valley College
... Why is one more acidic than another? The more acidic alcohol is the smaller alcohol (ie: 1). They have the fewest methyl groups pushing into the OH group. This causes the oxygen to pull as many electrons from the surrounding atoms as possible making the hydrogen more acidic (it leaves easier). As m ...
... Why is one more acidic than another? The more acidic alcohol is the smaller alcohol (ie: 1). They have the fewest methyl groups pushing into the OH group. This causes the oxygen to pull as many electrons from the surrounding atoms as possible making the hydrogen more acidic (it leaves easier). As m ...
Organic Synthesis - National Open University of Nigeria
... On oxidation with peroxy-acids, ketones are converted into esters or lactones. This reaction was discovered in 1899 by Baeyer and Villiger. Better yields are obtained with organic peroxy-acids such as perbenzoic acid, peracetic acid and trifluoroperacetic acid; although in practice nowadays most rea ...
... On oxidation with peroxy-acids, ketones are converted into esters or lactones. This reaction was discovered in 1899 by Baeyer and Villiger. Better yields are obtained with organic peroxy-acids such as perbenzoic acid, peracetic acid and trifluoroperacetic acid; although in practice nowadays most rea ...
Formose reaction controlled by boronic acid - Beilstein
... Figure 3 compares 1H and 13C NMR spectra for the products obtained in the presence of SPB and pVPB/NaSS. The 1H and 13C NMR spectra for SPB exhibit broad signals, similar to those for a formose reaction without boronic acid compounds. Since it was difficult to remove SPB from the reaction mixture, t ...
... Figure 3 compares 1H and 13C NMR spectra for the products obtained in the presence of SPB and pVPB/NaSS. The 1H and 13C NMR spectra for SPB exhibit broad signals, similar to those for a formose reaction without boronic acid compounds. Since it was difficult to remove SPB from the reaction mixture, t ...
Chapter 18 Carboxylic Acid Derivatives
... • Hydrolysis in aqueous acid is the reverse of Fischer esterification. – The role of the acid catalyst is to protonate the carbonyl oxygen and increase its electrophilic character toward attack by water (a weak nucleophile) to form a tetrahedral carbonyl addition intermediate. – Collapse of this int ...
... • Hydrolysis in aqueous acid is the reverse of Fischer esterification. – The role of the acid catalyst is to protonate the carbonyl oxygen and increase its electrophilic character toward attack by water (a weak nucleophile) to form a tetrahedral carbonyl addition intermediate. – Collapse of this int ...
Metal Carbenes
... Schrock later prepared a number of tantalum complexes including (Np)3Ta=CH(CMe3) and (η5Cp)2MeTa=CH2 ...
... Schrock later prepared a number of tantalum complexes including (Np)3Ta=CH(CMe3) and (η5Cp)2MeTa=CH2 ...
Naming Aldehydes & Ketones
... Cu2+ ions in an alkaline medium. • In these tests, the aldehyde group is oxidized to an acid by Cu2+ ions. O C R ...
... Cu2+ ions in an alkaline medium. • In these tests, the aldehyde group is oxidized to an acid by Cu2+ ions. O C R ...
ETHERS
... Mechanism of Cleavage — As seen below, the reaction is basically SN2 in base promoted cleavage. This means that the nucleophile attacks the backside (opposite the oxygen) of the less sterically hindered carbon preferentially – typical SN2 ...
... Mechanism of Cleavage — As seen below, the reaction is basically SN2 in base promoted cleavage. This means that the nucleophile attacks the backside (opposite the oxygen) of the less sterically hindered carbon preferentially – typical SN2 ...
Answers
... 1-propanol, CH3CH2CH2OH are all liquids at room temperature. When a liquid boils the intermolecular forces that hold the molecules in the liquid phase must be overcome to allow the molecules to enter the vapour phase. The stronger the intermolecular forces (for similar molecules) the higher the boil ...
... 1-propanol, CH3CH2CH2OH are all liquids at room temperature. When a liquid boils the intermolecular forces that hold the molecules in the liquid phase must be overcome to allow the molecules to enter the vapour phase. The stronger the intermolecular forces (for similar molecules) the higher the boil ...
Alcohols, Ethers, Aldehydes, and Ketones
... –OH and an –OR bound to the same carbon. NOTE: An oxygen in a ring structure is considered part of an –OR group. ...
... –OH and an –OR bound to the same carbon. NOTE: An oxygen in a ring structure is considered part of an –OR group. ...
Exercises Topic 8 - OCW
... thionyl chloride giving B which reacts with methylamine to give C. C is reduced with LiAlH4 to give D, a secondary amine (C3H9N). The second portion is treated with P2O5 to give E which is treated with D to give two products, F, an amide (C5H11NO), and A. The third portion is treated with methanol i ...
... thionyl chloride giving B which reacts with methylamine to give C. C is reduced with LiAlH4 to give D, a secondary amine (C3H9N). The second portion is treated with P2O5 to give E which is treated with D to give two products, F, an amide (C5H11NO), and A. The third portion is treated with methanol i ...
Alcohols and Phenols
... • Phenols (pKa ~10) are much more acidic than alcohols (pKa ~ 16) due to resonance stabilization of the phenoxide ion • Phenols react with NaOH solutions (but alcohols do not), forming soluble salts that are soluble in dilute aqueous • A phenolic component can be separated from an organic solution b ...
... • Phenols (pKa ~10) are much more acidic than alcohols (pKa ~ 16) due to resonance stabilization of the phenoxide ion • Phenols react with NaOH solutions (but alcohols do not), forming soluble salts that are soluble in dilute aqueous • A phenolic component can be separated from an organic solution b ...
Free Radical Chemistry and the Preparation of Alkyl
... Oxidations decrease e- density on C (formation of C-O, C-N or C-X bonds) so reactions that form alkyl halides (R-H to R-X) are oxidations ...
... Oxidations decrease e- density on C (formation of C-O, C-N or C-X bonds) so reactions that form alkyl halides (R-H to R-X) are oxidations ...
+ :O
... NOTE: Both of the above types of derivatives have intermolecular attractions similar to those of esters, and so they have boiling points in the same range as esters of comparable size. Both of these types of derivatives are important, powerful donors of their acyl groups and find much use in synthes ...
... NOTE: Both of the above types of derivatives have intermolecular attractions similar to those of esters, and so they have boiling points in the same range as esters of comparable size. Both of these types of derivatives are important, powerful donors of their acyl groups and find much use in synthes ...
Organic compounds containing Nitrogen
... with cuprous chloride and hydrogen chloride ,bromobenzene is formed with cuprous bromide and hydrogen bromide and benzonitrile with potassium cyanide and cuprous cyanide. These reactions are called Sandmeyer reactions. ...
... with cuprous chloride and hydrogen chloride ,bromobenzene is formed with cuprous bromide and hydrogen bromide and benzonitrile with potassium cyanide and cuprous cyanide. These reactions are called Sandmeyer reactions. ...
ppt
... Alkyl ammonium ions, R3NH+ X-, have pKa values in the range of 10-11 (ammonium ion, H4N+ X-, has a pKa ~ 9.3) The ammonium ions of aryl amines and heterocyclic aromatic amines are considerably more acidic than alkyl amines (pKa < 5). The nitrogen lone pair is less basic if it is in an sp2 hybridized ...
... Alkyl ammonium ions, R3NH+ X-, have pKa values in the range of 10-11 (ammonium ion, H4N+ X-, has a pKa ~ 9.3) The ammonium ions of aryl amines and heterocyclic aromatic amines are considerably more acidic than alkyl amines (pKa < 5). The nitrogen lone pair is less basic if it is in an sp2 hybridized ...
Wolff rearrangement

The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate product, which can undergo nucleophilic attack with weakly acidic nucleophiles such as water, alcohols, and amines, to generate carboxylic acid derivatives or undergo [2+2] cycloaddition reactions to form four-membered rings. The mechanism of the Wolff rearrangement has been the subject of debate since its first use. No single mechanism sufficiently describes the reaction, and there are often competing concerted and carbene-mediated pathways; for simplicity, only the textbook, concerted mechanism is shown below. The reaction was discovered by Ludwig Wolff in 1902. The Wolff rearrangement has great synthetic utility due to the accessibility of α-diazocarbonyl compounds, variety of reactions from the ketene intermediate, and stereochemical retention of the migrating group. However, the Wolff rearrangement has limitations due to the highly reactive nature of α-diazocarbonyl compounds, which can undergo a variety of competing reactions.The Wolff rearrangement can be induced via thermolysis, photolysis, or transition metal catalysis. In this last case, the reaction is sensitive to the transition metal; silver (I) oxide or other Ag(I) catalysts work well and are generally used. The Wolff rearrangement has been used in many total syntheses; the most common use is trapping the ketene intermediate with nucleophiles to form carboxylic acid derivatives. The Arndt-Eistert homologation is a specific example of this use, wherein a carboxylic acid may be elongated by a methylene unit. Another common use is in ring-contraction methods; if the α-diazo ketone is cyclic, the Wolff rearrangement results in a ring-contracted product. The Wolff rearrangement works well in generating ring-strained systems, where other reactions may fail.