Ethers, Sulfides, Epoxides
... In acid: protonate the oxygen, establishing the very good leaving group. More substituted carbon (more positive charge although more sterically hindered) is attacked by a weak nucleophile. H ...
... In acid: protonate the oxygen, establishing the very good leaving group. More substituted carbon (more positive charge although more sterically hindered) is attacked by a weak nucleophile. H ...
Carbonyl Compounds notes
... The NaOH in this reaction is not a catalyst; it is a reactant. The equilibrium can thus be moved further to the right by using the NaOH in excess. Saponification is thus a more efficient way of hydrolysing an ester than acid hydrolysis, since it results in a much better yield. After the mixture is c ...
... The NaOH in this reaction is not a catalyst; it is a reactant. The equilibrium can thus be moved further to the right by using the NaOH in excess. Saponification is thus a more efficient way of hydrolysing an ester than acid hydrolysis, since it results in a much better yield. After the mixture is c ...
Retrosynthesis - Organic Chemistry
... Work as many problems as you can, with this list of reactions in front of you if necessary, so that you can get through as many problems as you can without getting stuck on eth reagents/conditions, and so that you can learn and practice solving reaction problems. Use this list AFTER you have worked ...
... Work as many problems as you can, with this list of reactions in front of you if necessary, so that you can get through as many problems as you can without getting stuck on eth reagents/conditions, and so that you can learn and practice solving reaction problems. Use this list AFTER you have worked ...
Carbon-Carbon Bond Formation by Reductive
... Hydrocarbons: Although titanium (II) chlo ride in the form of complexes 1, 2 and 3 and ad mixed with a six to eight-fold excess of M e2AlCl is able to catalyze the polymerization of ethylene and other alpha-olefins [4], complex 1 in TH F or unsolvated TiCl2 suspended in toluene [6] caused neither ...
... Hydrocarbons: Although titanium (II) chlo ride in the form of complexes 1, 2 and 3 and ad mixed with a six to eight-fold excess of M e2AlCl is able to catalyze the polymerization of ethylene and other alpha-olefins [4], complex 1 in TH F or unsolvated TiCl2 suspended in toluene [6] caused neither ...
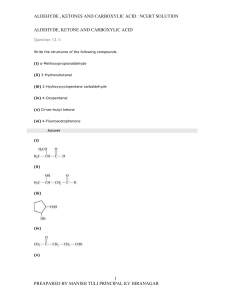
carbonyl compound group
... (vi) Oxime: Oximes are a class of organic compounds having the general formula RR′CNOH, where R is an organic side chain and R′ is either hydrogen or an organic side chain. If R′ is H, then it is known as aldoxime and if R′ is an organic side chain, it is known as ketoxime. ...
... (vi) Oxime: Oximes are a class of organic compounds having the general formula RR′CNOH, where R is an organic side chain and R′ is either hydrogen or an organic side chain. If R′ is H, then it is known as aldoxime and if R′ is an organic side chain, it is known as ketoxime. ...
Document
... 21.7: Preparation of Amines by Alkylation of Ammonia Ammonia and other alkylamines are good nucleophiles and react with 1° and 2° alkyl halides or tosylates via an SN2 reaction yielding alkyl amines. ...
... 21.7: Preparation of Amines by Alkylation of Ammonia Ammonia and other alkylamines are good nucleophiles and react with 1° and 2° alkyl halides or tosylates via an SN2 reaction yielding alkyl amines. ...
Drawing Organic Structures Functional Groups Constitutional Isomers
... • X eliminated from one carbon • H eliminated from adjacent carbon • Compete with substitution reactions ...
... • X eliminated from one carbon • H eliminated from adjacent carbon • Compete with substitution reactions ...
Elimination Reactions
... base (H2SO4, H3PO4). To favour SN1 over E1, use a good nucleophile. •To favour E2 over SN2, use a strong, bulky non-nucleophilic base. To favour SN2 over E2, use good nucleophiles that are relatively weak bases. •It is important to keep in mind that although you might choose reaction conditions that ...
... base (H2SO4, H3PO4). To favour SN1 over E1, use a good nucleophile. •To favour E2 over SN2, use a strong, bulky non-nucleophilic base. To favour SN2 over E2, use good nucleophiles that are relatively weak bases. •It is important to keep in mind that although you might choose reaction conditions that ...
Elimination Reactions
... base (H2SO4, H3PO4). To favour SN1 over E1, use a good nucleophile. •To favour E2 over SN2, use a strong, bulky non-nucleophilic base. To favour SN2 over E2, use good nucleophiles that are relatively weak bases. •It is important to keep in mind that although you might choose reaction conditions that ...
... base (H2SO4, H3PO4). To favour SN1 over E1, use a good nucleophile. •To favour E2 over SN2, use a strong, bulky non-nucleophilic base. To favour SN2 over E2, use good nucleophiles that are relatively weak bases. •It is important to keep in mind that although you might choose reaction conditions that ...
Organic Chemistry II Introduction
... Addition of HCN yields cyanohydrins 1° amines add to form imines, and 2° amines yield enamines Reaction with hydrazine gives hydrazones – Reduction of hydrazone in base yields an alkane – Reduction of hydrazone in acid/Zn yields an alkane Alcohols add to yield acetals Phosphoranes add to aldehydes a ...
... Addition of HCN yields cyanohydrins 1° amines add to form imines, and 2° amines yield enamines Reaction with hydrazine gives hydrazones – Reduction of hydrazone in base yields an alkane – Reduction of hydrazone in acid/Zn yields an alkane Alcohols add to yield acetals Phosphoranes add to aldehydes a ...
Chapter 24. Amines
... acceptors except in very strong acid • The C=O group is strongly electron-withdrawing, making the N a very weak base • Addition of a proton occurs on O but this destroys the double bond character of C=O as a requirement of stabilization by N ...
... acceptors except in very strong acid • The C=O group is strongly electron-withdrawing, making the N a very weak base • Addition of a proton occurs on O but this destroys the double bond character of C=O as a requirement of stabilization by N ...
Chapter 24. Amines
... acceptors except in very strong acid • The C=O group is strongly electron-withdrawing, making the N a very weak base • Addition of a proton occurs on O but this destroys the double bond character of C=O as a requirement of stabilization by N ...
... acceptors except in very strong acid • The C=O group is strongly electron-withdrawing, making the N a very weak base • Addition of a proton occurs on O but this destroys the double bond character of C=O as a requirement of stabilization by N ...
An Oxidation-Reduction Scheme: Borneol, Camphor, Isoborneol1
... sodium borohydride NaBH4, are widely used in reducing carbonyl groups. Lithium aluminum hydride, for example, reduces many compounds containing carbonyl groups, such as aldehydes, ketones, carboxylic acids, esters, or amides, whereas sodium borohydride reduces only aldehydes and ketones. The reduced ...
... sodium borohydride NaBH4, are widely used in reducing carbonyl groups. Lithium aluminum hydride, for example, reduces many compounds containing carbonyl groups, such as aldehydes, ketones, carboxylic acids, esters, or amides, whereas sodium borohydride reduces only aldehydes and ketones. The reduced ...
ppt
... the OH group of phenols cab participate in hydrogen bonding with other phenol molecules and to water. 24.4: Acidity of Phenols. Phenols are more acidic than aliphatic alcohols pKa ~ 16 H3CH2C O H ...
... the OH group of phenols cab participate in hydrogen bonding with other phenol molecules and to water. 24.4: Acidity of Phenols. Phenols are more acidic than aliphatic alcohols pKa ~ 16 H3CH2C O H ...
Chapter 1 Structure and Bonding
... a. LiAlH4 fully reduced the ester to an alcohol (similar to Grignard above) b. DIBAL reduces ester only to an aldehyde H O ...
... a. LiAlH4 fully reduced the ester to an alcohol (similar to Grignard above) b. DIBAL reduces ester only to an aldehyde H O ...
Reactions of Carboxylic Acids
... Sodium stearate has a polar end (the carboxylate end) and a nonpolar "tail“. The polar end is hydrophilic ("water-loving”). The nonpolar tail is hydrophobic ("water-hating”). In water, many stearate ions cluster together to form spherical aggregates; carboxylate ions are on the outside and nonpolar ...
... Sodium stearate has a polar end (the carboxylate end) and a nonpolar "tail“. The polar end is hydrophilic ("water-loving”). The nonpolar tail is hydrophobic ("water-hating”). In water, many stearate ions cluster together to form spherical aggregates; carboxylate ions are on the outside and nonpolar ...
α-cleavage of alkenes
... Alkanes: α-cleavage linear: peaks at 15, 29, 43, 57, etc. branched: predominate cleavage at branch points M+ often weak Alkenes: distinct M+ α-cleavage, fragments with mass CnH2n-1 and CnH2n (McLafferty) Cycloalkanes: prominent M+ fragments from loss of side groups ...
... Alkanes: α-cleavage linear: peaks at 15, 29, 43, 57, etc. branched: predominate cleavage at branch points M+ often weak Alkenes: distinct M+ α-cleavage, fragments with mass CnH2n-1 and CnH2n (McLafferty) Cycloalkanes: prominent M+ fragments from loss of side groups ...
Chapter 17 Allylic and Benzylic Reactivity
... The compounds that give the most stable carbocation intermediates are the ones that undergo the most rapid solvolysis. This problem deals with the effect of substituent on the stability of the carbocation intermediate. The key is to analyze the balance of resonance and polar substituent effects just ...
... The compounds that give the most stable carbocation intermediates are the ones that undergo the most rapid solvolysis. This problem deals with the effect of substituent on the stability of the carbocation intermediate. The key is to analyze the balance of resonance and polar substituent effects just ...
Microsoft Word - Open Access Repository of Indian Theses
... Stereoselective synthesis of (+)-(2R, 3R, 4R)-deacetylanisomycin Analogues of furanoses in which the ring oxygen is replaced by nitrogen and the anomeric hydroxyl is removed have been reported to be almost always inhibitors of the corresponding glycosidases. Glycosidases are involved in several impo ...
... Stereoselective synthesis of (+)-(2R, 3R, 4R)-deacetylanisomycin Analogues of furanoses in which the ring oxygen is replaced by nitrogen and the anomeric hydroxyl is removed have been reported to be almost always inhibitors of the corresponding glycosidases. Glycosidases are involved in several impo ...
Ch. 09 Alcohols, Ethers, Epoxides
... • A 1,2-shift can convert a less stable carbocation into a more stable carbocation. • Rearrangements are not unique to dehydration reactions. • Rearrangements can occur whenever a carbocation is formed as a reactive intermediate. • 2° carbocation A rearranges to the more stable 3° carbocation by a 1 ...
... • A 1,2-shift can convert a less stable carbocation into a more stable carbocation. • Rearrangements are not unique to dehydration reactions. • Rearrangements can occur whenever a carbocation is formed as a reactive intermediate. • 2° carbocation A rearranges to the more stable 3° carbocation by a 1 ...
aldehydes
... uncertain. As with DIBAH for aldehyde reductions, a low temperature (78 C) solvent (ether) is used to prevent further alkyl addition to the ketone to form an alcohol. (Acid chlorides are very good electrophiles). Carboxylic acids, esters, anhydrides and amides are not reduced by diorganocopper reag ...
... uncertain. As with DIBAH for aldehyde reductions, a low temperature (78 C) solvent (ether) is used to prevent further alkyl addition to the ketone to form an alcohol. (Acid chlorides are very good electrophiles). Carboxylic acids, esters, anhydrides and amides are not reduced by diorganocopper reag ...
Organic Chemistry II Introduction
... • The reaction with tertiary amines (R3N) gives an unstable species that cannot be isolated • HCl is neutralized by the amine or an added base ...
... • The reaction with tertiary amines (R3N) gives an unstable species that cannot be isolated • HCl is neutralized by the amine or an added base ...
Orbitals
... 14.11 Conjugate Nucleophilic Addition to α,βUnsaturated Aldehydes and Ketones Nucleophiles can add directly to carbonyl group of aldehydes and ketones, called 1,2-addition Nucleophiles can also add to conjugated C=C bond adjacent to carbonyl group of aldehydes and ketones, called conjugate addition ...
... 14.11 Conjugate Nucleophilic Addition to α,βUnsaturated Aldehydes and Ketones Nucleophiles can add directly to carbonyl group of aldehydes and ketones, called 1,2-addition Nucleophiles can also add to conjugated C=C bond adjacent to carbonyl group of aldehydes and ketones, called conjugate addition ...
Wolff rearrangement

The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate product, which can undergo nucleophilic attack with weakly acidic nucleophiles such as water, alcohols, and amines, to generate carboxylic acid derivatives or undergo [2+2] cycloaddition reactions to form four-membered rings. The mechanism of the Wolff rearrangement has been the subject of debate since its first use. No single mechanism sufficiently describes the reaction, and there are often competing concerted and carbene-mediated pathways; for simplicity, only the textbook, concerted mechanism is shown below. The reaction was discovered by Ludwig Wolff in 1902. The Wolff rearrangement has great synthetic utility due to the accessibility of α-diazocarbonyl compounds, variety of reactions from the ketene intermediate, and stereochemical retention of the migrating group. However, the Wolff rearrangement has limitations due to the highly reactive nature of α-diazocarbonyl compounds, which can undergo a variety of competing reactions.The Wolff rearrangement can be induced via thermolysis, photolysis, or transition metal catalysis. In this last case, the reaction is sensitive to the transition metal; silver (I) oxide or other Ag(I) catalysts work well and are generally used. The Wolff rearrangement has been used in many total syntheses; the most common use is trapping the ketene intermediate with nucleophiles to form carboxylic acid derivatives. The Arndt-Eistert homologation is a specific example of this use, wherein a carboxylic acid may be elongated by a methylene unit. Another common use is in ring-contraction methods; if the α-diazo ketone is cyclic, the Wolff rearrangement results in a ring-contracted product. The Wolff rearrangement works well in generating ring-strained systems, where other reactions may fail.