Organic Chemistry Introduction
... Anhydrous ammonia (NH3) is a liquid below -33 ºC Alkali metals dissolve in liquid ammonia Provide a solution of e- in NH3 Alkynes are reduced to trans alkenes with sodium or lithium in liquid ammonia ...
... Anhydrous ammonia (NH3) is a liquid below -33 ºC Alkali metals dissolve in liquid ammonia Provide a solution of e- in NH3 Alkynes are reduced to trans alkenes with sodium or lithium in liquid ammonia ...
Practice Problem - HCC Southeast Commons
... Only alkyl halides can be used (F, Cl, Br, I) – Aryl halides and vinylic halides do not react (their carbocations are too high in energy to form) ...
... Only alkyl halides can be used (F, Cl, Br, I) – Aryl halides and vinylic halides do not react (their carbocations are too high in energy to form) ...
Haloalkanes
... Benzvlic and aclylic halide are also quite reactive in SN2 reactions. In fact, they are even more reactive than primary and methyl halide. They can react by either an S N1 or SN2 mechanism. Besides being able to form relatively stable carbocations, simple allyl and benzyl substrate are sterically un ...
... Benzvlic and aclylic halide are also quite reactive in SN2 reactions. In fact, they are even more reactive than primary and methyl halide. They can react by either an S N1 or SN2 mechanism. Besides being able to form relatively stable carbocations, simple allyl and benzyl substrate are sterically un ...
United States Patent Dolphin et al.
... Commun., 1213-15 (1986). However, it has not been thought that the dihydroxy osmylation product of a p"p,'5 unsubstituted, meso-substituted porphyrin would be stable in view of the likelihood of rearrangement. Further, if the starting porphyrin bears a p,-substitution pattern, which lowers the overa ...
... Commun., 1213-15 (1986). However, it has not been thought that the dihydroxy osmylation product of a p"p,'5 unsubstituted, meso-substituted porphyrin would be stable in view of the likelihood of rearrangement. Further, if the starting porphyrin bears a p,-substitution pattern, which lowers the overa ...
An Efficient Method for Selective Deprotection of Trimethylsilyl
... reagents on various inorganic surfaces have recently attracted attention. The most advantage of these methods over conventional classical method is that they show cleaner reactions, decreased reaction time and straightforward workup. In continuation of our ongoing program to develop environmentally ...
... reagents on various inorganic surfaces have recently attracted attention. The most advantage of these methods over conventional classical method is that they show cleaner reactions, decreased reaction time and straightforward workup. In continuation of our ongoing program to develop environmentally ...
CHM238-01 EXAM 2 October 14, 2002 103
... that occurs under acidic conditions. Consider the situation shown below. (a) Provide arrows and necessary lone pairs to complete the reaction flow for the unexpected alcohol substitution reaction: H H3C ...
... that occurs under acidic conditions. Consider the situation shown below. (a) Provide arrows and necessary lone pairs to complete the reaction flow for the unexpected alcohol substitution reaction: H H3C ...
Organic Chemistry
... CH3 -C- O-C- CH 3 + H- O-H O H H Tetrahedral carbonyl addition intermediate ...
... CH3 -C- O-C- CH 3 + H- O-H O H H Tetrahedral carbonyl addition intermediate ...
chm238f02.exam2
... (b) Given pKas: CH3OH = 45; CH3OH = 16; H2 = 38; H2O = 16; PhOH = 10: Predict the direction and Keq of the reaction above. ...
... (b) Given pKas: CH3OH = 45; CH3OH = 16; H2 = 38; H2O = 16; PhOH = 10: Predict the direction and Keq of the reaction above. ...
Chapter 8 Lecture
... Reaction of hexyl bromide in a polar protic solvent required heating for 24 hours to form 76% of hexyl cyanide. With a polar aprotic solvent dimethyl sulfoxide and the less reactive hexyl chloride at room temperature for 20 minutes yielded 91 % of hexyl cyanide. ...
... Reaction of hexyl bromide in a polar protic solvent required heating for 24 hours to form 76% of hexyl cyanide. With a polar aprotic solvent dimethyl sulfoxide and the less reactive hexyl chloride at room temperature for 20 minutes yielded 91 % of hexyl cyanide. ...
Chapter 12- Alcohols from Carbonyl Compounds, Redox Reactions
... • Alkyl Sodium and alkyl potassium compounds are highly reactive and are among the most powerful bases • They react explosively with water and burst into flames when exposed to air • Organomercury and organolead compounds are much less reactive and more stable ...
... • Alkyl Sodium and alkyl potassium compounds are highly reactive and are among the most powerful bases • They react explosively with water and burst into flames when exposed to air • Organomercury and organolead compounds are much less reactive and more stable ...
Ketones and Aldehydes Reading: Wade chapter 18, sections 18
... Dipole-dipole interactions are the main intermolecular force holding ketones and aldehydes in the liquid phase. The dipole-dipole interaction is strong because of the high dipole moment attributable to the carbonyl group, giving ketones and aldehydes higher boiling points than hydrocarbons. However, ...
... Dipole-dipole interactions are the main intermolecular force holding ketones and aldehydes in the liquid phase. The dipole-dipole interaction is strong because of the high dipole moment attributable to the carbonyl group, giving ketones and aldehydes higher boiling points than hydrocarbons. However, ...
File
... used as an octane number enhancer in unleaded gasoline. It is prepared by the acid-catalyzed addition of methanol to 2-methylpropene ...
... used as an octane number enhancer in unleaded gasoline. It is prepared by the acid-catalyzed addition of methanol to 2-methylpropene ...
Some more basic organic (more naming, reactions, polymers)
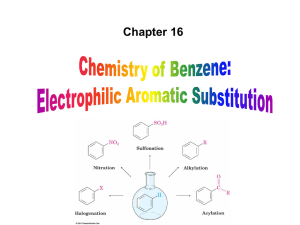
... pleasant odors. The most important compound in this family is benzene. ...
... pleasant odors. The most important compound in this family is benzene. ...
Aldehydes and Ketones
... carbonyl group always joined to at least one hydrogen (meaning it is always on the end!) ...
... carbonyl group always joined to at least one hydrogen (meaning it is always on the end!) ...
Carbon-Carbon Bond Formation by Reductive Coupling with
... Hydrocarbons: Although titanium (II) chlo ride in the form of complexes 1, 2 and 3 and ad mixed with a six to eight-fold excess of M e2AlCl is able to catalyze the polymerization of ethylene and other alpha-olefins [4], complex 1 in TH F or unsolvated TiCl2 suspended in toluene [6] caused neither ...
... Hydrocarbons: Although titanium (II) chlo ride in the form of complexes 1, 2 and 3 and ad mixed with a six to eight-fold excess of M e2AlCl is able to catalyze the polymerization of ethylene and other alpha-olefins [4], complex 1 in TH F or unsolvated TiCl2 suspended in toluene [6] caused neither ...
Chapter 12 –Part 2 Reaction of Carbonyl Compounds with
... t Any acidic hydrogen atoms in the carbonyl substrate will react ...
... t Any acidic hydrogen atoms in the carbonyl substrate will react ...
PHENOL - REACTIONS OF THE AROMATIC RING
... the OH group is electron releasing due to non-bonding pair donation it increases the electron density of the delocalised system it makes substitution much easier compared to benzene phenol reacts readily with bromine water WITHOUT A CATALYST it is so easy that multiple substitution takes place ...
... the OH group is electron releasing due to non-bonding pair donation it increases the electron density of the delocalised system it makes substitution much easier compared to benzene phenol reacts readily with bromine water WITHOUT A CATALYST it is so easy that multiple substitution takes place ...
Enantiodivergent conversion of chiral secondary alcohols into
... •Only works for aryl alcohols •Aryl boranes (e.g. Ph-9-BBN) incompatible due to protodeboronation during aqueous oxidative work-up •Indanol-derived carbamate gives same enantiomer (retention) with triethyl borane or ethylboronic acid (pyramidalization of geometrically constrained carbanion) ...
... •Only works for aryl alcohols •Aryl boranes (e.g. Ph-9-BBN) incompatible due to protodeboronation during aqueous oxidative work-up •Indanol-derived carbamate gives same enantiomer (retention) with triethyl borane or ethylboronic acid (pyramidalization of geometrically constrained carbanion) ...
Organometallic Compounds - Savita Pall and Chemistry
... synthesis reactions. Grignard reagents are often used to increase the length of the hydrocarbon chain in molecules. The Grignard reagents are useful in extending the length of the Carbon—chain in reaction pathways. The Grignard compounds are characterized by the presence of magnesium and a halogen g ...
... synthesis reactions. Grignard reagents are often used to increase the length of the hydrocarbon chain in molecules. The Grignard reagents are useful in extending the length of the Carbon—chain in reaction pathways. The Grignard compounds are characterized by the presence of magnesium and a halogen g ...
Chapter 18 Ketones and Aldehydes
... This molecule has two double bonds that might be formed by Wittig reactions. The central double bond could be formed in either of two ways. Both of these syntheses will probably work, and both will produce a mixture of cis and trans isomers. ...
... This molecule has two double bonds that might be formed by Wittig reactions. The central double bond could be formed in either of two ways. Both of these syntheses will probably work, and both will produce a mixture of cis and trans isomers. ...
main types and mechanisms of the reactions in organic chemistry
... elimination is formed. Product of reaction is secondary propanol (propan-2-ol). In hydration reaction proton attaches in accordance with Markovnikov’s rule – to the carbon atom with more hydrogen atoms, as electron dencity shifts to this atom because of positive inductive effect of СН3-group. Beside ...
... elimination is formed. Product of reaction is secondary propanol (propan-2-ol). In hydration reaction proton attaches in accordance with Markovnikov’s rule – to the carbon atom with more hydrogen atoms, as electron dencity shifts to this atom because of positive inductive effect of СН3-group. Beside ...
Presentation11_108
... Smaller carboxylic acids (1 to 4 carbons) are soluble in water Whereas the solubility of bigger carboxylic acids decrease with size Aromatic acids are insoluble in water ...
... Smaller carboxylic acids (1 to 4 carbons) are soluble in water Whereas the solubility of bigger carboxylic acids decrease with size Aromatic acids are insoluble in water ...
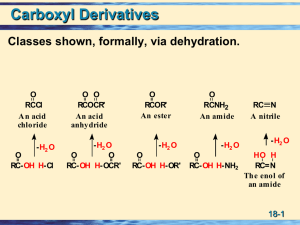
Chapter 18: Carboxylic Acids 18.1: Carboxylic Acid Nomenclature
... 18.4: Acidity of Carboxylic Acids. The pKa of carboxylic acids typically ~ 5. They are significantly more acidic than water or alcohols. Bronsted Acidity (Ch. 1.13): Carboxylic acids transfer a proton to water to give H3O+ and carboxylate anions, RCO2O R ...
... 18.4: Acidity of Carboxylic Acids. The pKa of carboxylic acids typically ~ 5. They are significantly more acidic than water or alcohols. Bronsted Acidity (Ch. 1.13): Carboxylic acids transfer a proton to water to give H3O+ and carboxylate anions, RCO2O R ...
10. Alkyl Halides
... The E2 ( for elimination, bimolecular) reaction is the most commonly occurring pathway for elimination. It is closely analogous to the SN2 mechanism. The rxn rate = k x [RX][Base] The essential feature of the E2 mechanism is that it is a one step process without intermediate. As the attacking ba ...
... The E2 ( for elimination, bimolecular) reaction is the most commonly occurring pathway for elimination. It is closely analogous to the SN2 mechanism. The rxn rate = k x [RX][Base] The essential feature of the E2 mechanism is that it is a one step process without intermediate. As the attacking ba ...
Wolff rearrangement

The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate product, which can undergo nucleophilic attack with weakly acidic nucleophiles such as water, alcohols, and amines, to generate carboxylic acid derivatives or undergo [2+2] cycloaddition reactions to form four-membered rings. The mechanism of the Wolff rearrangement has been the subject of debate since its first use. No single mechanism sufficiently describes the reaction, and there are often competing concerted and carbene-mediated pathways; for simplicity, only the textbook, concerted mechanism is shown below. The reaction was discovered by Ludwig Wolff in 1902. The Wolff rearrangement has great synthetic utility due to the accessibility of α-diazocarbonyl compounds, variety of reactions from the ketene intermediate, and stereochemical retention of the migrating group. However, the Wolff rearrangement has limitations due to the highly reactive nature of α-diazocarbonyl compounds, which can undergo a variety of competing reactions.The Wolff rearrangement can be induced via thermolysis, photolysis, or transition metal catalysis. In this last case, the reaction is sensitive to the transition metal; silver (I) oxide or other Ag(I) catalysts work well and are generally used. The Wolff rearrangement has been used in many total syntheses; the most common use is trapping the ketene intermediate with nucleophiles to form carboxylic acid derivatives. The Arndt-Eistert homologation is a specific example of this use, wherein a carboxylic acid may be elongated by a methylene unit. Another common use is in ring-contraction methods; if the α-diazo ketone is cyclic, the Wolff rearrangement results in a ring-contracted product. The Wolff rearrangement works well in generating ring-strained systems, where other reactions may fail.