+ Br2, FeBr3 + Br2, FeBr3
... (b) What is the reactive electrophile in the above reaction? NO2+, nitronium ion. (c) If we used only pure (fuming) sulfuric acid, what would be the product(s)? mostly sulfonation of Cl benzene, both o and p, because SO3H+ becomes the superelectrophile and there is not as much protons for the dehydr ...
... (b) What is the reactive electrophile in the above reaction? NO2+, nitronium ion. (c) If we used only pure (fuming) sulfuric acid, what would be the product(s)? mostly sulfonation of Cl benzene, both o and p, because SO3H+ becomes the superelectrophile and there is not as much protons for the dehydr ...
Chpt 23Final7e
... nitration reaction. This leads to the desired product with the hydroxy group in the 3 position. Step (1) Oxidation at a benzylic carbon (Section 20.6A) can be brought about using chromic acid to give benzoic acid. Step (2) Nitration of the aromatic ring using HNO3 in H2 SO4 . The meta-directing carb ...
... nitration reaction. This leads to the desired product with the hydroxy group in the 3 position. Step (1) Oxidation at a benzylic carbon (Section 20.6A) can be brought about using chromic acid to give benzoic acid. Step (2) Nitration of the aromatic ring using HNO3 in H2 SO4 . The meta-directing carb ...
Chloroperbenzoic_aci..
... Epoxidations of alkenes with m-CPBA are usually carried out by mixing the reactants in CH2 Cl2 or CHCl3 at 0–25 ◦ C.9 After the reaction is complete the reaction mixture is cooled in an ice bath and the precipitated m-chlorobenzoic acid is removed by filtration. The organic layer is washed with sodi ...
... Epoxidations of alkenes with m-CPBA are usually carried out by mixing the reactants in CH2 Cl2 or CHCl3 at 0–25 ◦ C.9 After the reaction is complete the reaction mixture is cooled in an ice bath and the precipitated m-chlorobenzoic acid is removed by filtration. The organic layer is washed with sodi ...
Conjugate addition_Clayden
... The reason that α,β-unsaturated carbonyl compounds react differently is conjugation, the phenomenon we discussed in Chapter 7. There we introduced you to the idea that bringing two π systems (two C=C bonds, for example, or a C=C bond and a C=O bond) close together leads to a stabilizing interaction. ...
... The reason that α,β-unsaturated carbonyl compounds react differently is conjugation, the phenomenon we discussed in Chapter 7. There we introduced you to the idea that bringing two π systems (two C=C bonds, for example, or a C=C bond and a C=O bond) close together leads to a stabilizing interaction. ...
2009 Final Exam - Department of Chemistry | Oregon State University
... Consider a "General Chemistry Battery" in which one beaker contains aqueous tin sulfate (SnSO4) and a tin metal electrode and the other beaker contains aqueous lead sulfate (PbSO4) and a lead metal electrode. Which of the following statements is false? (A) (B) (C) (D) ...
... Consider a "General Chemistry Battery" in which one beaker contains aqueous tin sulfate (SnSO4) and a tin metal electrode and the other beaker contains aqueous lead sulfate (PbSO4) and a lead metal electrode. Which of the following statements is false? (A) (B) (C) (D) ...
(Z)-Tamoxifen and Tetrasubstituted Alkenes and Dienes via a Regio
... π-orbitals in the addition component. Thus the poor result with the methylmagnesium chloride (entry 8) is due to the inefficiency of the carbometalation and not to the subsequent cross-coupling. The yield of the Grignard additions to propargyl alcohols can be enhanced, in some cases, by the addition ...
... π-orbitals in the addition component. Thus the poor result with the methylmagnesium chloride (entry 8) is due to the inefficiency of the carbometalation and not to the subsequent cross-coupling. The yield of the Grignard additions to propargyl alcohols can be enhanced, in some cases, by the addition ...
Document
... – Reduction of an aldehyde gives a primary alcohol (-CH2OH). – Reduction of a ketone gives a secondary alcohol (-CHOH-). ...
... – Reduction of an aldehyde gives a primary alcohol (-CH2OH). – Reduction of a ketone gives a secondary alcohol (-CHOH-). ...
Alcohols - WordPress.com
... Alcohols are weak Brønsted bases Protonated by strong acids to yield oxonium ions, ...
... Alcohols are weak Brønsted bases Protonated by strong acids to yield oxonium ions, ...
Photoremovable Protecting Groups
... compounds, that are employed in biochemistry and other biological studies has also appeared. In general, photolysis reactions present a noteworthy and often ideal alternative to all other methods for introducing reagents or substrates into reactions or biological media. The ability to control the sp ...
... compounds, that are employed in biochemistry and other biological studies has also appeared. In general, photolysis reactions present a noteworthy and often ideal alternative to all other methods for introducing reagents or substrates into reactions or biological media. The ability to control the sp ...
Chapter 12 Carboxylic Acids
... concentrated acetic acid causes acid burns when it comes into contact with the skin. Dissociation of either an acid or an alcohol involves breaking an O-H bond, but dissociation of a carboxylic acid gives a carboxylate ion with the negative charge spread out equally over two oxygen atoms, compared w ...
... concentrated acetic acid causes acid burns when it comes into contact with the skin. Dissociation of either an acid or an alcohol involves breaking an O-H bond, but dissociation of a carboxylic acid gives a carboxylate ion with the negative charge spread out equally over two oxygen atoms, compared w ...
Asymmetric Glycine Enolate Aldol Reactions
... David A. Evans* and Ann E. Weber2 Contribution from the Department of Chemistry, Haruard University, Cambridge, Massachusetts 02138. Received May I , 1986 ...
... David A. Evans* and Ann E. Weber2 Contribution from the Department of Chemistry, Haruard University, Cambridge, Massachusetts 02138. Received May I , 1986 ...
Alkene-Addn-PartB-2012-ques
... character; more stable transition state (left) has positive charge on more highly substituted carbon ...
... character; more stable transition state (left) has positive charge on more highly substituted carbon ...
Ketones and Aldehydes
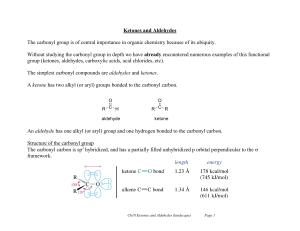
... Without studying the carbonyl group in depth we have already encountered numerous examples of this functional group (ketones, aldehydes, carboxylic acids, acid chlorides, etc). The simplest carbonyl compounds are aldehydes and ketones. A ketone has two alkyl (or aryl) groups bonded to the carbonyl c ...
... Without studying the carbonyl group in depth we have already encountered numerous examples of this functional group (ketones, aldehydes, carboxylic acids, acid chlorides, etc). The simplest carbonyl compounds are aldehydes and ketones. A ketone has two alkyl (or aryl) groups bonded to the carbonyl c ...
Chapter - FIU Faculty Websites
... • Acetal formation is catalyzed by acids, such as TsOH. • Because conversion of an aldehyde or ketone to an acetal is a reversible reaction, an acetal can be hydrolyzed to an aldehyde or ketone by treatment with aqueous acid (but are stable in basic solutions). ...
... • Acetal formation is catalyzed by acids, such as TsOH. • Because conversion of an aldehyde or ketone to an acetal is a reversible reaction, an acetal can be hydrolyzed to an aldehyde or ketone by treatment with aqueous acid (but are stable in basic solutions). ...
Carbonyl Compounds I. Aldehydes and Ketones
... the angle strain means that a sizable enhancement of both the reactivity and equilibrium constant for addition is expected. In practice, the strain effect is so large that cyclopropanone reacts rapidly with methanol to give a stable hemiketal from which the ketone cannot be recovered. Cyclobutanone ...
... the angle strain means that a sizable enhancement of both the reactivity and equilibrium constant for addition is expected. In practice, the strain effect is so large that cyclopropanone reacts rapidly with methanol to give a stable hemiketal from which the ketone cannot be recovered. Cyclobutanone ...
evans enolate alkylation
... oxazolidinone auxiliary. There are several protocols for this, but just a couple will be given. Carbonyl Hydrolysis: The amide like carbonyl should be more susceptible to nucleophilic attack, than the carbamate carbonyl, so HO- should be able to hydrolyze this to a carboxylic acid. When it’s done, L ...
... oxazolidinone auxiliary. There are several protocols for this, but just a couple will be given. Carbonyl Hydrolysis: The amide like carbonyl should be more susceptible to nucleophilic attack, than the carbamate carbonyl, so HO- should be able to hydrolyze this to a carboxylic acid. When it’s done, L ...
Ir-catalysed formation of C− F bonds. From allylic alcohols to α
... mixture of 5 : 1. Less water failed to dissolve SelectF, and more water increased by-product (3a) formation. The catalyst loading could be lowered to 1 mol% (Table 2, entry 1). At acidic pH, higher amounts of unwanted ketone 3a are obtained and the reaction is complete in a shorter time (15 min) (Ta ...
... mixture of 5 : 1. Less water failed to dissolve SelectF, and more water increased by-product (3a) formation. The catalyst loading could be lowered to 1 mol% (Table 2, entry 1). At acidic pH, higher amounts of unwanted ketone 3a are obtained and the reaction is complete in a shorter time (15 min) (Ta ...
Caboxylic acid Derivatives
... Acid Catalyzed Nucleophilic Acyl Substitution In the previous examples, a nucleophile attacked the carbonyl group to generate a tetrahedral intermediate. However some nucleophiles are too weak to directly attack the carbonyl group (especially in the less reactive acid derivatives). E.g. an alcohol ...
... Acid Catalyzed Nucleophilic Acyl Substitution In the previous examples, a nucleophile attacked the carbonyl group to generate a tetrahedral intermediate. However some nucleophiles are too weak to directly attack the carbonyl group (especially in the less reactive acid derivatives). E.g. an alcohol ...
Reactions of Alcohols
... The ZnCl2 coordinates to the hydroxyl oxygen, and this generates a far superior leaving group. Primary alcohols react in a similar fashion except the free cation is not generated, and the substitution is of S N2 ...
... The ZnCl2 coordinates to the hydroxyl oxygen, and this generates a far superior leaving group. Primary alcohols react in a similar fashion except the free cation is not generated, and the substitution is of S N2 ...
Chapter-1 ALCOHOLS
... is regioselective. The Grignard reagent attacks at the least substituted side of the carbon-oxygen bonds, if there is one. ...
... is regioselective. The Grignard reagent attacks at the least substituted side of the carbon-oxygen bonds, if there is one. ...
13. amines - WordPress.com
... Basicity of amines is related to their structure. Basic character of an amine depends upon the ease of formation of the cation by accepting a proton from the acid. As the stability of the cation increases, the basicity also increases. a) Comparison of basicity of alkyl amines (alkanamines) and ammon ...
... Basicity of amines is related to their structure. Basic character of an amine depends upon the ease of formation of the cation by accepting a proton from the acid. As the stability of the cation increases, the basicity also increases. a) Comparison of basicity of alkyl amines (alkanamines) and ammon ...
Wolff rearrangement

The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate product, which can undergo nucleophilic attack with weakly acidic nucleophiles such as water, alcohols, and amines, to generate carboxylic acid derivatives or undergo [2+2] cycloaddition reactions to form four-membered rings. The mechanism of the Wolff rearrangement has been the subject of debate since its first use. No single mechanism sufficiently describes the reaction, and there are often competing concerted and carbene-mediated pathways; for simplicity, only the textbook, concerted mechanism is shown below. The reaction was discovered by Ludwig Wolff in 1902. The Wolff rearrangement has great synthetic utility due to the accessibility of α-diazocarbonyl compounds, variety of reactions from the ketene intermediate, and stereochemical retention of the migrating group. However, the Wolff rearrangement has limitations due to the highly reactive nature of α-diazocarbonyl compounds, which can undergo a variety of competing reactions.The Wolff rearrangement can be induced via thermolysis, photolysis, or transition metal catalysis. In this last case, the reaction is sensitive to the transition metal; silver (I) oxide or other Ag(I) catalysts work well and are generally used. The Wolff rearrangement has been used in many total syntheses; the most common use is trapping the ketene intermediate with nucleophiles to form carboxylic acid derivatives. The Arndt-Eistert homologation is a specific example of this use, wherein a carboxylic acid may be elongated by a methylene unit. Another common use is in ring-contraction methods; if the α-diazo ketone is cyclic, the Wolff rearrangement results in a ring-contracted product. The Wolff rearrangement works well in generating ring-strained systems, where other reactions may fail.