Reactions of Alkyl Halides (SN1, SN2, E1, and E2 reactions)
... The difference in the rate of reaction of alcohols with conc. HCl is used as a qualitative test (the Lucas Test) to determine the degree of substitution of an alcohol. Lucas reagent is conc. HCl, saturated with ZnCl2 salt. The Zn+2 ion coordinates (bonds) with the alcohol oxygen even better than ...
... The difference in the rate of reaction of alcohols with conc. HCl is used as a qualitative test (the Lucas Test) to determine the degree of substitution of an alcohol. Lucas reagent is conc. HCl, saturated with ZnCl2 salt. The Zn+2 ion coordinates (bonds) with the alcohol oxygen even better than ...
Chapter 19
... Amine salts are also used to catalyze a variety of organic reactions that feature two components that are soluble in different liquid phases (e.g. organic and aqueous) ...
... Amine salts are also used to catalyze a variety of organic reactions that feature two components that are soluble in different liquid phases (e.g. organic and aqueous) ...
Organic Chemistry
... Organic chemistry looks at “oxidation” differently than we discussed in redox reactions where “oxidation” was all about losing electrons. In Organic Chemistry, “oxidation” is all about gaining OXYGEN! The more oxygen attached to the carbon, the more “oxidized” the carbon is considered. ...
... Organic chemistry looks at “oxidation” differently than we discussed in redox reactions where “oxidation” was all about losing electrons. In Organic Chemistry, “oxidation” is all about gaining OXYGEN! The more oxygen attached to the carbon, the more “oxidized” the carbon is considered. ...
PDF carboxylic acids
... have only one COOH functional group. Compounds 5 and 6 possess hydroxyl as well as carboxylic groups. In such cases they are named as hydroxyl derivatives of carboxylic acids rather than carboxyl derivative of alcohols. The presence of a double bond in the main chain is represented by ending the nam ...
... have only one COOH functional group. Compounds 5 and 6 possess hydroxyl as well as carboxylic groups. In such cases they are named as hydroxyl derivatives of carboxylic acids rather than carboxyl derivative of alcohols. The presence of a double bond in the main chain is represented by ending the nam ...
Synthesis of Heterocycles from Anthranilic acid
... Anthranilic acid (2-aminobenzoic acid, Aa) is the biochemical precursor to the amino acid tryptophan, as well as a catabolic product of tryptophan in animals. It is also integrated into many alkaloids isolated from plants. Aa is produced industrially for production of dyestuffs and pharmaceuticals. ...
... Anthranilic acid (2-aminobenzoic acid, Aa) is the biochemical precursor to the amino acid tryptophan, as well as a catabolic product of tryptophan in animals. It is also integrated into many alkaloids isolated from plants. Aa is produced industrially for production of dyestuffs and pharmaceuticals. ...
+ ∂ - CHEM171 – Lecture Series Seven : 2012/05
... Have a cation and an anion forming. If a carbon-carbon bond breaks in this fashion then we have a carbocation and a carbanion resulting CHEM171 – Lecture Series Seven : 2012/02 ...
... Have a cation and an anion forming. If a carbon-carbon bond breaks in this fashion then we have a carbocation and a carbanion resulting CHEM171 – Lecture Series Seven : 2012/02 ...
lecture 7 reductive eliminations
... • In all oxidative additions, a pair of electrons from the metal is used to break the A−B bond in the reagent. • In the SN2 pathway, adopted for polarized A‐B substrates such as alkyl halides, the metal electron pair of LnM directly attacks the A–B σ* orbital by an in‐line attack at the least electr ...
... • In all oxidative additions, a pair of electrons from the metal is used to break the A−B bond in the reagent. • In the SN2 pathway, adopted for polarized A‐B substrates such as alkyl halides, the metal electron pair of LnM directly attacks the A–B σ* orbital by an in‐line attack at the least electr ...
Organic Synthesis II
... Mechanisms for many oxidation reactions (even well-known ones) are significantly more complex than drawn throughout this course (and in many cases are not known or understood). Some are based on factual mechanistic data; some should be treated more as a mnemonic than explanation. ...
... Mechanisms for many oxidation reactions (even well-known ones) are significantly more complex than drawn throughout this course (and in many cases are not known or understood). Some are based on factual mechanistic data; some should be treated more as a mnemonic than explanation. ...
Slide 1
... HCl (g)) causes ester formation (esterification) along with dehydration. The equilibrium constant is not large (Keq ~ 1) but high yields can be obtained by adding a large excess of one of the reactants and removing the H2O formed. The reaction is reversible. A large excess of H2O favors the reverse ...
... HCl (g)) causes ester formation (esterification) along with dehydration. The equilibrium constant is not large (Keq ~ 1) but high yields can be obtained by adding a large excess of one of the reactants and removing the H2O formed. The reaction is reversible. A large excess of H2O favors the reverse ...
Lectures 4-6
... - effective for the conversion of 1° alcohols to RCO2H and 2° alcohols to ketones - oxidizes multiple bonds and 1,2-diols. ...
... - effective for the conversion of 1° alcohols to RCO2H and 2° alcohols to ketones - oxidizes multiple bonds and 1,2-diols. ...
Chapter 25 Alt Notes 0910
... Because the -OH group is quite polar, the properties of alcohols depend upon the number of -OH groups per molecule and the size of the organic group. The boiling points of monohydric alcohols increase with increasing molecular weight. The solubility of monohydric alcohols in water decrease wit ...
... Because the -OH group is quite polar, the properties of alcohols depend upon the number of -OH groups per molecule and the size of the organic group. The boiling points of monohydric alcohols increase with increasing molecular weight. The solubility of monohydric alcohols in water decrease wit ...
Neuman Chapter - Department of Chemistry
... This E1 mechanism is analogous to the two-step SN1 substitution mechanism (Chapter 7). The "1" in E1 indicates that the rate determining step of the reaction is unimolecular. This rate determining step is the ionization step (the first step) that involves only the haloalkane substrate (RX). The E1 r ...
... This E1 mechanism is analogous to the two-step SN1 substitution mechanism (Chapter 7). The "1" in E1 indicates that the rate determining step of the reaction is unimolecular. This rate determining step is the ionization step (the first step) that involves only the haloalkane substrate (RX). The E1 r ...
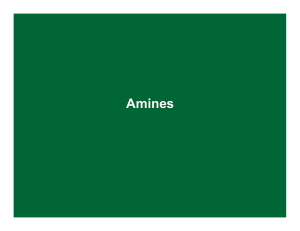
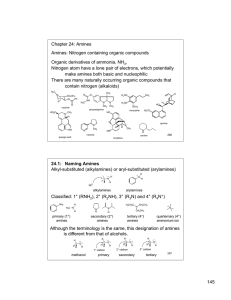
145 Chapter 24: Amines Amines: Nitrogen containing organic
... Table 24.1 (p. 899): pKa values of ammonium ions Alkyl ammonium ions, R3NH+ X-, have pKa values in the range of 10-11 (ammonium ion, H4N+ X-, has a pKa ~ 9.25) The ammonium ions of aryl amines and heterocyclic aromatic amines are considerably less basic than alkyl amines (pKa ~ 5 or less). The nitr ...
... Table 24.1 (p. 899): pKa values of ammonium ions Alkyl ammonium ions, R3NH+ X-, have pKa values in the range of 10-11 (ammonium ion, H4N+ X-, has a pKa ~ 9.25) The ammonium ions of aryl amines and heterocyclic aromatic amines are considerably less basic than alkyl amines (pKa ~ 5 or less). The nitr ...
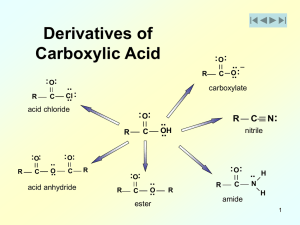
carboxylic acids and their derivatives
... Exercise 18-1 Explain why the proton line position of the acidic hydrogen of a carboxylic acid, dissolved in a nonpolar solvent such as carbon tetrachloride, changes much less with concentration than does that of the OH proton of an alcohol under the same conditions (Section 9-1OE). ...
... Exercise 18-1 Explain why the proton line position of the acidic hydrogen of a carboxylic acid, dissolved in a nonpolar solvent such as carbon tetrachloride, changes much less with concentration than does that of the OH proton of an alcohol under the same conditions (Section 9-1OE). ...
9: Formation of Alkenes and Alkynes. Elimination Reactions
... This E1 mechanism is analogous to the two-step SN1 substitution mechanism (Chapter 7). The "1" in E1 indicates that the rate determining step of the reaction is unimolecular. This rate determining step is the ionization step (the first step) that involves only the haloalkane substrate (RX). The E1 r ...
... This E1 mechanism is analogous to the two-step SN1 substitution mechanism (Chapter 7). The "1" in E1 indicates that the rate determining step of the reaction is unimolecular. This rate determining step is the ionization step (the first step) that involves only the haloalkane substrate (RX). The E1 r ...
Selective Incorporation of Difluoromethylene
... Cy2 as dative ligand. A range of functionalities were tolerated under these reaction conditions. It is noteworthy that both aryl bromide and aryl chloride could be efficiently transformed with low catalyst loading even in large scale. Intriguingly, the base-induced cleavage of the α-aryl-α,αdifluoro ...
... Cy2 as dative ligand. A range of functionalities were tolerated under these reaction conditions. It is noteworthy that both aryl bromide and aryl chloride could be efficiently transformed with low catalyst loading even in large scale. Intriguingly, the base-induced cleavage of the α-aryl-α,αdifluoro ...
Document
... • When a mixture of stereoisomers is possible from a dehydrohalogenation, the major product is the more stable stereoisomer. • A reaction is stereoselective when it forms predominantly or exclusively one stereoisomer when two or more are possible. • The E2 reaction is stereoselective because one ste ...
... • When a mixture of stereoisomers is possible from a dehydrohalogenation, the major product is the more stable stereoisomer. • A reaction is stereoselective when it forms predominantly or exclusively one stereoisomer when two or more are possible. • The E2 reaction is stereoselective because one ste ...
19.7 Reversible Addition Reactions of Aldehydes and Ketones
... selective than LiAlH4 • LiAlH4 reacts with alkyl halides, alkyl tosylates, and nitro groups, but NaBH4 does not ...
... selective than LiAlH4 • LiAlH4 reacts with alkyl halides, alkyl tosylates, and nitro groups, but NaBH4 does not ...
File
... H CH3 H CF3 destabilizes the δ+ of the carbonyl, H CH3 G G+H-hydrate making the CF3 carbonyl higher in energy than the aldehyde. This makes the hydrate reaction of the CF3 carbonyl downhill, resulting in a higher amount of hydrate. 16. In each pair, predict which would have a greater percentage of h ...
... H CH3 H CF3 destabilizes the δ+ of the carbonyl, H CH3 G G+H-hydrate making the CF3 carbonyl higher in energy than the aldehyde. This makes the hydrate reaction of the CF3 carbonyl downhill, resulting in a higher amount of hydrate. 16. In each pair, predict which would have a greater percentage of h ...
23.3 Carbonyl Compounds
... • As a result, only weak attractions hold ester molecules to one another. – Esters have much lower boiling points than carboxylic acids. ...
... • As a result, only weak attractions hold ester molecules to one another. – Esters have much lower boiling points than carboxylic acids. ...
View/Open - AURA - Alfred University
... that triflic acid is not responsible for the progress of the reaction, suggests that PEDOT can mediate the Ritter reaction under mild conditions and it may now be possible to use unprotected, acid-sensitive functional groups. In an attempt to determine the scope of the PEDOT-mediated Ritter reactio ...
... that triflic acid is not responsible for the progress of the reaction, suggests that PEDOT can mediate the Ritter reaction under mild conditions and it may now be possible to use unprotected, acid-sensitive functional groups. In an attempt to determine the scope of the PEDOT-mediated Ritter reactio ...
Anhydrides, Esters and Amides
... • In practice what occurs if the two are mixed is an acidbase reaction to form an ammonium salt. • If this salt is heated to a high enough temperature, water is eliminated and an amide forms. O CH3 C-OH + H2 NCH2 CH3 ...
... • In practice what occurs if the two are mixed is an acidbase reaction to form an ammonium salt. • If this salt is heated to a high enough temperature, water is eliminated and an amide forms. O CH3 C-OH + H2 NCH2 CH3 ...
Wolff rearrangement

The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate product, which can undergo nucleophilic attack with weakly acidic nucleophiles such as water, alcohols, and amines, to generate carboxylic acid derivatives or undergo [2+2] cycloaddition reactions to form four-membered rings. The mechanism of the Wolff rearrangement has been the subject of debate since its first use. No single mechanism sufficiently describes the reaction, and there are often competing concerted and carbene-mediated pathways; for simplicity, only the textbook, concerted mechanism is shown below. The reaction was discovered by Ludwig Wolff in 1902. The Wolff rearrangement has great synthetic utility due to the accessibility of α-diazocarbonyl compounds, variety of reactions from the ketene intermediate, and stereochemical retention of the migrating group. However, the Wolff rearrangement has limitations due to the highly reactive nature of α-diazocarbonyl compounds, which can undergo a variety of competing reactions.The Wolff rearrangement can be induced via thermolysis, photolysis, or transition metal catalysis. In this last case, the reaction is sensitive to the transition metal; silver (I) oxide or other Ag(I) catalysts work well and are generally used. The Wolff rearrangement has been used in many total syntheses; the most common use is trapping the ketene intermediate with nucleophiles to form carboxylic acid derivatives. The Arndt-Eistert homologation is a specific example of this use, wherein a carboxylic acid may be elongated by a methylene unit. Another common use is in ring-contraction methods; if the α-diazo ketone is cyclic, the Wolff rearrangement results in a ring-contracted product. The Wolff rearrangement works well in generating ring-strained systems, where other reactions may fail.