The Carbonyl Group Nomenclature of Aldehydes and Ketones
... that are in between those of alcohols and hydrocarbons of the same molecular weight: – Alcohols form hydrogen bonds, and have high boiling points. – Hydrocarbons are nonpolar, and have low boiling points. – Aldehydes and ketones are polar, so they have higher boiling points than hydrocarbons, but th ...
... that are in between those of alcohols and hydrocarbons of the same molecular weight: – Alcohols form hydrogen bonds, and have high boiling points. – Hydrocarbons are nonpolar, and have low boiling points. – Aldehydes and ketones are polar, so they have higher boiling points than hydrocarbons, but th ...
CHAPTER V Fischer-Tropsch Syncrude
... hydrocarbons (>C20), do not have a single α-value, but two. The first α-value (α1) describes the carbon number distribution from C3 to C12, while the second α-value (α2) describes the distribution of the C20 and heavier fraction.(4) This seems to be a mathematical convenience, since the α-value seem ...
... hydrocarbons (>C20), do not have a single α-value, but two. The first α-value (α1) describes the carbon number distribution from C3 to C12, while the second α-value (α2) describes the distribution of the C20 and heavier fraction.(4) This seems to be a mathematical convenience, since the α-value seem ...
Ch 7 - Practice problem (Answers)
... Chapter 7 Structure and Preparation of Alkenes - Elimination Reactions: Answers ...
... Chapter 7 Structure and Preparation of Alkenes - Elimination Reactions: Answers ...
Amide bond formation and peptide coupling
... support (collagen). Amides also play a key role for medicinal chemists. An in-depth analysis of the Comprehensive Medicinal Chemistry database revealed that the carboxamide group appears in more than 25% of known drugs.1 This can be expected, since carboxamides are neutral, are stable and have both ...
... support (collagen). Amides also play a key role for medicinal chemists. An in-depth analysis of the Comprehensive Medicinal Chemistry database revealed that the carboxamide group appears in more than 25% of known drugs.1 This can be expected, since carboxamides are neutral, are stable and have both ...
Organic Chemistry
... You can imagine complicated R' groups that may be much more difficult to name as alkyl or aryl groups than those we have shown in Figures 15.30 and 15.32. When faced with naming such complicated molecules, organic chemists often consult specialized books on organic nomenclature. However, many ester ...
... You can imagine complicated R' groups that may be much more difficult to name as alkyl or aryl groups than those we have shown in Figures 15.30 and 15.32. When faced with naming such complicated molecules, organic chemists often consult specialized books on organic nomenclature. However, many ester ...
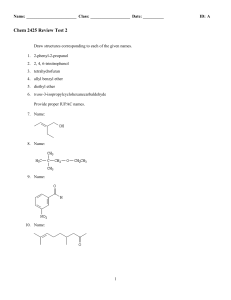
Chem 2425-Test 2 Review
... A highly useful and general method for the synthesis of alcohols is the addition of Grignard reagents to carbonyl compounds. Show what Grignard reagent and what carbonyl compound you would start with to prepare each alcohol below. List all possibilities. ...
... A highly useful and general method for the synthesis of alcohols is the addition of Grignard reagents to carbonyl compounds. Show what Grignard reagent and what carbonyl compound you would start with to prepare each alcohol below. List all possibilities. ...
Synthesis of Alcohols Using Grignard Reagents Grignard reagents
... O What remains is the combination of Grignard reagent and carbonyl compound that can be used to prepare the alcohol. ...
... O What remains is the combination of Grignard reagent and carbonyl compound that can be used to prepare the alcohol. ...
university of london thesis
... The chapter covering results and discussion opens with a brief overview o f the investigational work previously carried out into the Sn I like ring opening o f a model molecule, 1-methylcyclohexene oxide. This is followed by a description o f how a new methodology for the acidic ring opening o f epo ...
... The chapter covering results and discussion opens with a brief overview o f the investigational work previously carried out into the Sn I like ring opening o f a model molecule, 1-methylcyclohexene oxide. This is followed by a description o f how a new methodology for the acidic ring opening o f epo ...
Chemistry 217 Problem Set 3 Recommended Problems from the Book
... 5. IR spectroscopy is commonly used by crime labs to determine the type of fiber found at a crime scene. Explain how you could use IR to distinguish between the following synthetic fibers. Note that these fibers are all polymers made up of repeating units between the brackets. Nylon contains an amid ...
... 5. IR spectroscopy is commonly used by crime labs to determine the type of fiber found at a crime scene. Explain how you could use IR to distinguish between the following synthetic fibers. Note that these fibers are all polymers made up of repeating units between the brackets. Nylon contains an amid ...
lec-3- 211( Elim+ Re..
... Oxidation is the beginning of the deterioration process. Think of how a slice of apple turns brown when exposed to air. Oxidation leads to the formation of free radicals which are unstable molecules in the body that have one unpaired electron. They can cause oxidation and damage to the cells. This ...
... Oxidation is the beginning of the deterioration process. Think of how a slice of apple turns brown when exposed to air. Oxidation leads to the formation of free radicals which are unstable molecules in the body that have one unpaired electron. They can cause oxidation and damage to the cells. This ...
Chapter 15
... OH’s attached to the same carbon) that is nucleophilic and can attack another molecule of chromic acid to form a second chromate ester. Removal of the second proton forms the carboxylic acid. H ...
... OH’s attached to the same carbon) that is nucleophilic and can attack another molecule of chromic acid to form a second chromate ester. Removal of the second proton forms the carboxylic acid. H ...
synthetic approaches for quinoline and isoquinoline
... of a benzene ring fused to a pyridine ring5 . In a broader sense, the term isoquinoline is used to make reference to isoquinoline derivatives.1‐Benzylisoquinoline is the structural backbone in naturally occurring alkaloids including papaverine and morphine. The isoquino ...
... of a benzene ring fused to a pyridine ring5 . In a broader sense, the term isoquinoline is used to make reference to isoquinoline derivatives.1‐Benzylisoquinoline is the structural backbone in naturally occurring alkaloids including papaverine and morphine. The isoquino ...
Ch14b: Carboxylic Acids
... effect makes it difficult to use in many cases. ‣ Phenols and carboxylic acids are acidic, but other substances with these functional groups don’t have the same side effect. ‣ In salicylic acid, the functional groups interact to create that greater acidity. ...
... effect makes it difficult to use in many cases. ‣ Phenols and carboxylic acids are acidic, but other substances with these functional groups don’t have the same side effect. ‣ In salicylic acid, the functional groups interact to create that greater acidity. ...
Chapter 19. Aldehydes and Ketones: Nucleophilic Addition Reactions
... Nu- approaches 45° to the plane of C=O and adds ...
... Nu- approaches 45° to the plane of C=O and adds ...
Chapter 19. Aldehydes and Ketones: Nucleophilic Addition Reactions
... Nu- approaches 45° to the plane of C=O and adds ...
... Nu- approaches 45° to the plane of C=O and adds ...
Studies toward the Stereoselective Synthesis of the
... Mycotoxins are toxic secondary metabolites produced by fungi and they are the causative agents of various diseases in man and his domestic animals. Human beings and animals get the diseases, commonly called mycotoxicoses through the ingestion of foods or feeds contaminated by these toxic fungal meta ...
... Mycotoxins are toxic secondary metabolites produced by fungi and they are the causative agents of various diseases in man and his domestic animals. Human beings and animals get the diseases, commonly called mycotoxicoses through the ingestion of foods or feeds contaminated by these toxic fungal meta ...
عرض تقديمي من PowerPoint
... Heterocyclic analogues of Cyclopropane All three membered rings have one major property in common- a strained ring which confers great reactivity on the compounds in comparison with their openchain analogues. ...
... Heterocyclic analogues of Cyclopropane All three membered rings have one major property in common- a strained ring which confers great reactivity on the compounds in comparison with their openchain analogues. ...
lecture 6 oxidative addition
... • In all oxidative additions, a pair of electrons from the metal is used to break the A−B bond in the reagent. • In the SN2 pathway, adopted for polarized A‐B substrates such as alkyl halides, the metal electron pair of LnM directly attacks the A–B σ* orbital by an in‐line attack at the least electr ...
... • In all oxidative additions, a pair of electrons from the metal is used to break the A−B bond in the reagent. • In the SN2 pathway, adopted for polarized A‐B substrates such as alkyl halides, the metal electron pair of LnM directly attacks the A–B σ* orbital by an in‐line attack at the least electr ...
Aldehydes and Ketones
... • The negative part of the added molecule adds to the positive carbonyl carbon • The positive part of the added molecule adds to the negative carbonyl oxygen • d+ d- d+ d• -C=O + X-Y -C-O-X ...
... • The negative part of the added molecule adds to the positive carbonyl carbon • The positive part of the added molecule adds to the negative carbonyl oxygen • d+ d- d+ d• -C=O + X-Y -C-O-X ...
Document
... It is important to know what nucleophiles will add to carbonyl groups. • Cl¯, Br¯ and I¯ are good nucleophiles in substitution reactions at sp3 hybridized carbons, but they are ineffective nucleophiles in addition. • When these nucleophiles add to a carbonyl, they cleave the C—O bond, forming an a ...
... It is important to know what nucleophiles will add to carbonyl groups. • Cl¯, Br¯ and I¯ are good nucleophiles in substitution reactions at sp3 hybridized carbons, but they are ineffective nucleophiles in addition. • When these nucleophiles add to a carbonyl, they cleave the C—O bond, forming an a ...
Organic Chemistry - UCR Chemistry
... and compounds related to them. These are ionic reactions in which one group on the molecule (a leaving group) is replaced by another group (a nucleophile). The transformation of haloalkanes (R-X) into alcohols (R-OH) where an OH group replaces the halogen (X) is an example of nucleophilic substituti ...
... and compounds related to them. These are ionic reactions in which one group on the molecule (a leaving group) is replaced by another group (a nucleophile). The transformation of haloalkanes (R-X) into alcohols (R-OH) where an OH group replaces the halogen (X) is an example of nucleophilic substituti ...
Alkyl Halides02
... This is also a qualitative test for identifying alcohols, i.e, the Lucas test. HCl with ZnCl 2 catalyst are used. 3 alcohols react quickly, 2 slowly, and 1 don’t react. Note that 1 and 2 alcohols will react with special reagents to produce alkyl halides (i.e., thionyl chloride, SOCl2, or PBr3) ...
... This is also a qualitative test for identifying alcohols, i.e, the Lucas test. HCl with ZnCl 2 catalyst are used. 3 alcohols react quickly, 2 slowly, and 1 don’t react. Note that 1 and 2 alcohols will react with special reagents to produce alkyl halides (i.e., thionyl chloride, SOCl2, or PBr3) ...
PowerPoint ******
... “Wagner-Meerwein Rearrangements” must form the final products that are thermodynamically more stable than the starting materials. Some processes proceeding do appear to require uphill steps (formation of less stable carbocation). ...
... “Wagner-Meerwein Rearrangements” must form the final products that are thermodynamically more stable than the starting materials. Some processes proceeding do appear to require uphill steps (formation of less stable carbocation). ...
Wolff rearrangement

The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate product, which can undergo nucleophilic attack with weakly acidic nucleophiles such as water, alcohols, and amines, to generate carboxylic acid derivatives or undergo [2+2] cycloaddition reactions to form four-membered rings. The mechanism of the Wolff rearrangement has been the subject of debate since its first use. No single mechanism sufficiently describes the reaction, and there are often competing concerted and carbene-mediated pathways; for simplicity, only the textbook, concerted mechanism is shown below. The reaction was discovered by Ludwig Wolff in 1902. The Wolff rearrangement has great synthetic utility due to the accessibility of α-diazocarbonyl compounds, variety of reactions from the ketene intermediate, and stereochemical retention of the migrating group. However, the Wolff rearrangement has limitations due to the highly reactive nature of α-diazocarbonyl compounds, which can undergo a variety of competing reactions.The Wolff rearrangement can be induced via thermolysis, photolysis, or transition metal catalysis. In this last case, the reaction is sensitive to the transition metal; silver (I) oxide or other Ag(I) catalysts work well and are generally used. The Wolff rearrangement has been used in many total syntheses; the most common use is trapping the ketene intermediate with nucleophiles to form carboxylic acid derivatives. The Arndt-Eistert homologation is a specific example of this use, wherein a carboxylic acid may be elongated by a methylene unit. Another common use is in ring-contraction methods; if the α-diazo ketone is cyclic, the Wolff rearrangement results in a ring-contracted product. The Wolff rearrangement works well in generating ring-strained systems, where other reactions may fail.