
Hein and Arena - faculty at Chemeketa
... electropositive Mg to form a strongly polar covalent bond. As a result the charge distribution in the Grignard reagent is such that the organic group (R) is partially negative and the –MgX group is partially positive. This charge distribution directs the manner in which Grignard reacts with other co ...
... electropositive Mg to form a strongly polar covalent bond. As a result the charge distribution in the Grignard reagent is such that the organic group (R) is partially negative and the –MgX group is partially positive. This charge distribution directs the manner in which Grignard reacts with other co ...
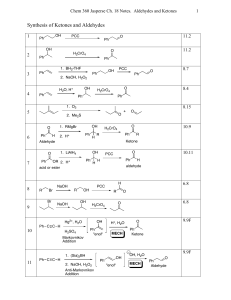
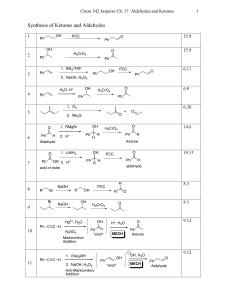
Synthesis of Ketones and Aldehydes
... Classification of Mechanisms Associated With Ketone/Aldehyde Reactions. • There may seem to be a dizzying number of mechanisms this chapter. But all of them simplify into some combination of acid- or base-catalyzed addition reaction, elimination reaction and/or substitution reaction. • To predict wh ...
... Classification of Mechanisms Associated With Ketone/Aldehyde Reactions. • There may seem to be a dizzying number of mechanisms this chapter. But all of them simplify into some combination of acid- or base-catalyzed addition reaction, elimination reaction and/or substitution reaction. • To predict wh ...
Electrophilic Aromatic Substitution and Substituted Benzenes
... substitution. • The first step involves addition of the electrophile (E+) to form a resonance stabilized carbocation. • The Hammond postulate makes it possible to predict the relative rate of the reaction by looking at the stability of the carbocation ...
... substitution. • The first step involves addition of the electrophile (E+) to form a resonance stabilized carbocation. • The Hammond postulate makes it possible to predict the relative rate of the reaction by looking at the stability of the carbocation ...
Document
... substitution. • The first step involves addition of the electrophile (E+) to form a resonance stabilized carbocation. • The Hammond postulate makes it possible to predict the relative rate of the reaction by looking at the stability of the carbocation ...
... substitution. • The first step involves addition of the electrophile (E+) to form a resonance stabilized carbocation. • The Hammond postulate makes it possible to predict the relative rate of the reaction by looking at the stability of the carbocation ...
Carbonyls
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
Reductive Elimination
... All these elimination reactions can be thought of as being related to oxidative additions of a C−H bond to the metal. This is seen more clearly for β elimination if we write the metalacyclopropane (X2) form of the alkene hydride product, and for α elimination if we consider the X2 form for the produ ...
... All these elimination reactions can be thought of as being related to oxidative additions of a C−H bond to the metal. This is seen more clearly for β elimination if we write the metalacyclopropane (X2) form of the alkene hydride product, and for α elimination if we consider the X2 form for the produ ...
Ch17 Lecture
... • The amide melatonin is thought to induce sleep because its production is increased by the body during the evening. ...
... • The amide melatonin is thought to induce sleep because its production is increased by the body during the evening. ...
Organometallic Compounds: Alkyllithium Reagent
... Grignard reagents react with formaldehyde to give a primary alcohol ...
... Grignard reagents react with formaldehyde to give a primary alcohol ...
ENZYME MIMIC ASYMMETRIC ALDOL REACTIONS
... The mechanism of proline-catalyzed intermolecular aldol reaction has been extensively studied. Again, the key enamine intermediate is formed by the secondary amine and the ketone donor. This enamine then attacks the aldehyde acceptor activated by a ...
... The mechanism of proline-catalyzed intermolecular aldol reaction has been extensively studied. Again, the key enamine intermediate is formed by the secondary amine and the ketone donor. This enamine then attacks the aldehyde acceptor activated by a ...
Carboxylic acids from primary alcohols and aldehydes by a
... Downloaded by: IP-Proxy CONSORTIUM:OHIOLINK (OhioLink - Small Institutions) , OhioLink - Small Institutions. Copyrighted material. ...
... Downloaded by: IP-Proxy CONSORTIUM:OHIOLINK (OhioLink - Small Institutions) , OhioLink - Small Institutions. Copyrighted material. ...
© John Congleton, Orange Coast College Organic Chemistry 220
... Be able to predict whether a reaction will proceed via o SN1 and E1 o S N2 o SN2 and E2 o E2 What makes a good nucleophile? What makes a good base? What makes a good leaving group? What is meant by high and low polarizability? Allylic bromination Understand, be able to predict, and be able to comple ...
... Be able to predict whether a reaction will proceed via o SN1 and E1 o S N2 o SN2 and E2 o E2 What makes a good nucleophile? What makes a good base? What makes a good leaving group? What is meant by high and low polarizability? Allylic bromination Understand, be able to predict, and be able to comple ...
Learning Guide for Chapter 9 - Alkyl Halides I
... How do the strength of the nucleophile and electrophile fit together? SN2: strong Nu, weak E SN1: weak Nu, strong E Scenario A: strong Nu added to alkyl halide Nu attacks! SN2 Scenario B: weak Nu added to alkyl halide Nu can't attack, sits around waiting until alkyl halide dissociates, then attacks ...
... How do the strength of the nucleophile and electrophile fit together? SN2: strong Nu, weak E SN1: weak Nu, strong E Scenario A: strong Nu added to alkyl halide Nu attacks! SN2 Scenario B: weak Nu added to alkyl halide Nu can't attack, sits around waiting until alkyl halide dissociates, then attacks ...
- University at Albany
... Also, alkyl halide reactivity decreases from methyl to 10 to 20 to 30. In fact, 30 alkyl halides do not react by SN2. Leaving group: The substrate should have a good leaving group. A good leaving group should be electron withdrawing, relatively stable, and polarizable. They are weak bases. Examples ...
... Also, alkyl halide reactivity decreases from methyl to 10 to 20 to 30. In fact, 30 alkyl halides do not react by SN2. Leaving group: The substrate should have a good leaving group. A good leaving group should be electron withdrawing, relatively stable, and polarizable. They are weak bases. Examples ...
Synthesis of Ketones and Aldehydes
... Classification of Mechanisms Associated With Ketone/Aldehyde Reactions. • There may seem to be a dizzying number of mechanisms this chapter. But all of them simplify into some combination of acid- or base-catalyzed addition reaction, elimination reaction and/or substitution reaction. • To predict wh ...
... Classification of Mechanisms Associated With Ketone/Aldehyde Reactions. • There may seem to be a dizzying number of mechanisms this chapter. But all of them simplify into some combination of acid- or base-catalyzed addition reaction, elimination reaction and/or substitution reaction. • To predict wh ...
Chapter 9
... Aromatic rings can be sulfonated in the laboratory by reaction with fuming sulfuric acid, a mixture of H2SO4 and SO3 • The reactive electrophile is either HSO3+ or neutral SO3 • Substitution occurs by the same two-step mechanism seen for bromination and nitration • Aromatic sulfonation does not occu ...
... Aromatic rings can be sulfonated in the laboratory by reaction with fuming sulfuric acid, a mixture of H2SO4 and SO3 • The reactive electrophile is either HSO3+ or neutral SO3 • Substitution occurs by the same two-step mechanism seen for bromination and nitration • Aromatic sulfonation does not occu ...
organic chemistry reaction scheme
... *Note: Lithium aluminium hydride (or Lithium tetrahydridoaluminate(III)), LiAlH4, is one of the few reagents that can reduce an acid to an alcohol; the initial product is an alkoxide which the alcohol is liberated by hydrolysis. The –H ion acts as a nucleophile, and can attack the carbon atom of the ...
... *Note: Lithium aluminium hydride (or Lithium tetrahydridoaluminate(III)), LiAlH4, is one of the few reagents that can reduce an acid to an alcohol; the initial product is an alkoxide which the alcohol is liberated by hydrolysis. The –H ion acts as a nucleophile, and can attack the carbon atom of the ...
Review of Organic Chem II
... 3. The types of intermediates involved (cation, anion, or radical) should be consistent with the reaction classification above a. If the reaction is cationic, don’t show anionic intermediates b. If the reaction is anionic, don’t show cationic intermediates 4. Usually conditions are ionic. 5. Use a r ...
... 3. The types of intermediates involved (cation, anion, or radical) should be consistent with the reaction classification above a. If the reaction is cationic, don’t show anionic intermediates b. If the reaction is anionic, don’t show cationic intermediates 4. Usually conditions are ionic. 5. Use a r ...
GRIGNARD REAGENTS
... The structures on either side of a straight two-headed arrow are resonance forms of the same chemical entity; they differ only the location of electrons, and can be interconverted by the movement of curly arrows. A carbonyl group is a HYBRID of the two resonance forms shown. ...
... The structures on either side of a straight two-headed arrow are resonance forms of the same chemical entity; they differ only the location of electrons, and can be interconverted by the movement of curly arrows. A carbonyl group is a HYBRID of the two resonance forms shown. ...
ch15[1].
... Characteristic Reactions • In the general reaction, we showed the nucleophile as an anion; this need not be the case. • Neutral molecules such as water, alcohols, ammonia, and amines can also serve as nucleophiles. • In the general reaction, we showed the leaving group as an anion to illustrate an ...
... Characteristic Reactions • In the general reaction, we showed the nucleophile as an anion; this need not be the case. • Neutral molecules such as water, alcohols, ammonia, and amines can also serve as nucleophiles. • In the general reaction, we showed the leaving group as an anion to illustrate an ...
Aromatic Compounds
... • Addition of a reagent such as HCl to an alkene • The electrophilic hydrogen approaches the p electrons of ...
... • Addition of a reagent such as HCl to an alkene • The electrophilic hydrogen approaches the p electrons of ...
Alcohols and Phenols
... • Phenols (pKa ~10) are much more acidic than alcohols (pKa ~ 16) due to resonance stabilization of the phenoxide ion • Phenols react with NaOH solutions (but alcohols do not), forming soluble salts that are soluble in dilute aqueous • A phenolic component can be separated from an organic solution b ...
... • Phenols (pKa ~10) are much more acidic than alcohols (pKa ~ 16) due to resonance stabilization of the phenoxide ion • Phenols react with NaOH solutions (but alcohols do not), forming soluble salts that are soluble in dilute aqueous • A phenolic component can be separated from an organic solution b ...
Chapter 15 Carboxylic Acids and Esters
... • The ester functional group is a key structural feature in fats, oils, and other lipids. • The ester functional group is also found in fruits and flowers. • Many esters are fragrant and represent some of nature’s most pleasant odors. • Esters are commonly used as flavoring agents in foods and scent ...
... • The ester functional group is a key structural feature in fats, oils, and other lipids. • The ester functional group is also found in fruits and flowers. • Many esters are fragrant and represent some of nature’s most pleasant odors. • Esters are commonly used as flavoring agents in foods and scent ...
Wolff rearrangement

The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate product, which can undergo nucleophilic attack with weakly acidic nucleophiles such as water, alcohols, and amines, to generate carboxylic acid derivatives or undergo [2+2] cycloaddition reactions to form four-membered rings. The mechanism of the Wolff rearrangement has been the subject of debate since its first use. No single mechanism sufficiently describes the reaction, and there are often competing concerted and carbene-mediated pathways; for simplicity, only the textbook, concerted mechanism is shown below. The reaction was discovered by Ludwig Wolff in 1902. The Wolff rearrangement has great synthetic utility due to the accessibility of α-diazocarbonyl compounds, variety of reactions from the ketene intermediate, and stereochemical retention of the migrating group. However, the Wolff rearrangement has limitations due to the highly reactive nature of α-diazocarbonyl compounds, which can undergo a variety of competing reactions.The Wolff rearrangement can be induced via thermolysis, photolysis, or transition metal catalysis. In this last case, the reaction is sensitive to the transition metal; silver (I) oxide or other Ag(I) catalysts work well and are generally used. The Wolff rearrangement has been used in many total syntheses; the most common use is trapping the ketene intermediate with nucleophiles to form carboxylic acid derivatives. The Arndt-Eistert homologation is a specific example of this use, wherein a carboxylic acid may be elongated by a methylene unit. Another common use is in ring-contraction methods; if the α-diazo ketone is cyclic, the Wolff rearrangement results in a ring-contracted product. The Wolff rearrangement works well in generating ring-strained systems, where other reactions may fail.


















![ch15[1].](http://s1.studyres.com/store/data/008194241_2-0a33cfb98ac502873dac865380b726e0-300x300.png)


