Atomic nuclei: radioactivity and types of radiation
... particles or electromagnetic waves. These emitted particles or electromagnetic waves are called radiation. When a nucleus undergoes radioactive decay, it emits radiation and the nucleus is said to be radioactive. We are exposed to small amounts of radiation all the time. Even the rocks around us emi ...
... particles or electromagnetic waves. These emitted particles or electromagnetic waves are called radiation. When a nucleus undergoes radioactive decay, it emits radiation and the nucleus is said to be radioactive. We are exposed to small amounts of radiation all the time. Even the rocks around us emi ...
nuclear fission
... very radioactive product which they thought was a nucleus of a very heavy element, formed when the neutrons joined onto the uranium nucleus. However, when they analysed the products they found not elements heavier than uranium but two about half the mass number of the original uranium, a solution th ...
... very radioactive product which they thought was a nucleus of a very heavy element, formed when the neutrons joined onto the uranium nucleus. However, when they analysed the products they found not elements heavier than uranium but two about half the mass number of the original uranium, a solution th ...
Nuclear Physics and Radioactivity2
... At the beginning of the century (1900), not much was known about the atom. The electron was discovered by J.J. Thomson in 1897. Thomson did not know much was known about the electron and advanced the "plum pudding" model of the atom. The number of electrons contained in an atom could not be determin ...
... At the beginning of the century (1900), not much was known about the atom. The electron was discovered by J.J. Thomson in 1897. Thomson did not know much was known about the electron and advanced the "plum pudding" model of the atom. The number of electrons contained in an atom could not be determin ...
FUSION AND FISSION
... • The fusion of two nuclei lighter than iron or nickel generally releases energy. • The fusion of nuclei heavier than them absorbs energy. Result: gain or loss of energy ...
... • The fusion of two nuclei lighter than iron or nickel generally releases energy. • The fusion of nuclei heavier than them absorbs energy. Result: gain or loss of energy ...
section_2_review_set
... creates the chemical bonds 3. What is the claim to fame for the neutron? stabilizes the nucleus 4. What is the mass of each of the following particles?: proton 1; neutron 1; electron 0. 5. What is the charge for each of the following particles?: proton +1; neutron 0; electron -1. 6. What two things ...
... creates the chemical bonds 3. What is the claim to fame for the neutron? stabilizes the nucleus 4. What is the mass of each of the following particles?: proton 1; neutron 1; electron 0. 5. What is the charge for each of the following particles?: proton +1; neutron 0; electron -1. 6. What two things ...
The Nature of Molecules
... • Diagram of typical atomic structure: • Atomic #/mass of: H, He, C, O, N, S, P, Ne ...
... • Diagram of typical atomic structure: • Atomic #/mass of: H, He, C, O, N, S, P, Ne ...
Alpha
... 4. Which of the three radioactive emissions best fit the following statements? Write the correct symbol/s on the lines. a) These emissions are charged. ____________ b) This emission is the most massive (heaviest). ____________ c) This emission is the most charged. ____________ d) This emission is mo ...
... 4. Which of the three radioactive emissions best fit the following statements? Write the correct symbol/s on the lines. a) These emissions are charged. ____________ b) This emission is the most massive (heaviest). ____________ c) This emission is the most charged. ____________ d) This emission is mo ...
Chapter 3 Nuclear Radiation
... Which of the following radioisotopes are most likely to be used in nuclear medicine? ...
... Which of the following radioisotopes are most likely to be used in nuclear medicine? ...
Chapter 3 Nuclear Radiation
... Which of the following radioisotopes are most likely to be used in nuclear medicine? ...
... Which of the following radioisotopes are most likely to be used in nuclear medicine? ...
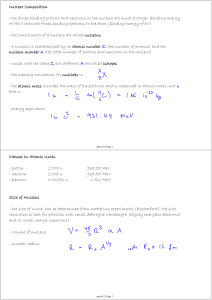
Masses in Atomic Units - proton 1.007 u 938.28 MeV
... of MeV) than the forces binding electrons to the atom (binding energy of eV) - the constituents of a nucleus are called nucleons - a nucleus is characterized by its atomic number Z (the number of protons) and the nucleon number A (the total number of protons and neutrons in the nucleus) - nuclei wit ...
... of MeV) than the forces binding electrons to the atom (binding energy of eV) - the constituents of a nucleus are called nucleons - a nucleus is characterized by its atomic number Z (the number of protons) and the nucleon number A (the total number of protons and neutrons in the nucleus) - nuclei wit ...
Chapter 2 - Speedway High School
... Essential Elements of Life • Carbon, hydrogen, oxygen, and nitrogen make up 96% of living matter • Trace elements are those required by an organism in minute quantities ...
... Essential Elements of Life • Carbon, hydrogen, oxygen, and nitrogen make up 96% of living matter • Trace elements are those required by an organism in minute quantities ...
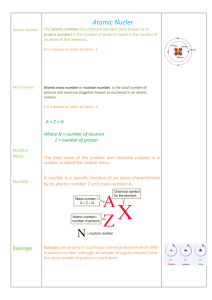
Atomic Nuclei - RAJEEV Classes
... number increases by 1 but mass number remains same. c)The emission of a γ-particle does not change the mass number or the atomic number of the radioactive nucleus. The γ-particle emission by a radioactive nucleus lowers its energy state. ...
... number increases by 1 but mass number remains same. c)The emission of a γ-particle does not change the mass number or the atomic number of the radioactive nucleus. The γ-particle emission by a radioactive nucleus lowers its energy state. ...
8.P.1.1 Warm-Up Questions for Website
... 189. Which of these is a physical change that takes place in a garden? A. Insects eat the leaves of a plant for food. B. Flowers grow after a summer rain shower. C. Earthworms loosen soil as they travel through it. D. Leaves convert sunlight to sugar by ...
... 189. Which of these is a physical change that takes place in a garden? A. Insects eat the leaves of a plant for food. B. Flowers grow after a summer rain shower. C. Earthworms loosen soil as they travel through it. D. Leaves convert sunlight to sugar by ...
Geologic Dating! - rgreenbergscience
... • The natural forces that form sedimentary rock can also reveal fossils that have been hidden in layers of rock for millions of years. • As forces within the earth uplift rock and weathering forces wear away rock, fossils become exposed. • Remember: A fossil is evidence (remains or traces) of an org ...
... • The natural forces that form sedimentary rock can also reveal fossils that have been hidden in layers of rock for millions of years. • As forces within the earth uplift rock and weathering forces wear away rock, fossils become exposed. • Remember: A fossil is evidence (remains or traces) of an org ...
Radiation_What Is It
... During negative beta decay, excess neutrons are converted into protons, electrons, and antineutrinos. The protons remain in the nucleus but the new electrons are emitted as negative beta particles (ß-) or negatrons. You may wish to think of them as “nuclear electrons.” ...
... During negative beta decay, excess neutrons are converted into protons, electrons, and antineutrinos. The protons remain in the nucleus but the new electrons are emitted as negative beta particles (ß-) or negatrons. You may wish to think of them as “nuclear electrons.” ...
Chapter 6: Chemistry in Biology
... Substances that release hydrogen ions ( H ) when dissolved in water are called __________. Substances that release hydroxide ions ( OH ) when dissolved in water are called __________. pH and Buffers: The measure of concentration of H in a solution is called __________. ...
... Substances that release hydrogen ions ( H ) when dissolved in water are called __________. Substances that release hydroxide ions ( OH ) when dissolved in water are called __________. pH and Buffers: The measure of concentration of H in a solution is called __________. ...
entc 4390 medical imaging
... The initial and final energy states of the nucleus are said to be isomers. A common form of isomeric transition is gamma decay (g) in which the energy is released as a packet of energy (a quantum or photon) termed a gamma (g) ray An isomeric transition that competes with gamma decay is internal conv ...
... The initial and final energy states of the nucleus are said to be isomers. A common form of isomeric transition is gamma decay (g) in which the energy is released as a packet of energy (a quantum or photon) termed a gamma (g) ray An isomeric transition that competes with gamma decay is internal conv ...
6.2 - Hockerill Students
... It is the strong nuclear force that holds the nucleons together, but this is a very short range force. ...
... It is the strong nuclear force that holds the nucleons together, but this is a very short range force. ...
Candidate 2 - Elgin Academy
... (Am) is a substance made by scientists and has an atomic number 95. The nuclide notation for americium is: ...
... (Am) is a substance made by scientists and has an atomic number 95. The nuclide notation for americium is: ...
Lanthanides and actinides are elements of the inner transition series
... Any of the 14 rare earth elements from lanthanum to lutetium in the periodic table. Because their outermost orbitals are empty, they have very similar chemistry. Below them are the actinides. ...
... Any of the 14 rare earth elements from lanthanum to lutetium in the periodic table. Because their outermost orbitals are empty, they have very similar chemistry. Below them are the actinides. ...
Chapter 29
... • These forces should cause the nucleus to fly apart • The nuclei are stable because of the presence of another, short-range force, called the nuclear force • This is an attractive force that acts between all nuclear particles • The nuclear attractive force is stronger than the Coulomb repulsive for ...
... • These forces should cause the nucleus to fly apart • The nuclei are stable because of the presence of another, short-range force, called the nuclear force • This is an attractive force that acts between all nuclear particles • The nuclear attractive force is stronger than the Coulomb repulsive for ...
Chapter 4.3: How Atoms Differ
... Radioactive atoms undergo _____________ that can alter their ___________ through __________ reactions. ...
... Radioactive atoms undergo _____________ that can alter their ___________ through __________ reactions. ...