chap7_nucleus
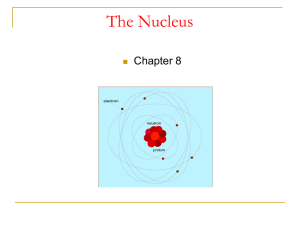
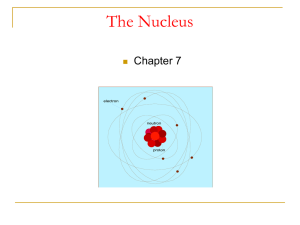
... Rutherford found that the only way to explain the deflections was to picture an atom with a tiny nucleus in which positive charge existed and nearly all the mass existed; And the electrons were some distance away from the nucleus. In other words, AN ATOM IS MOSTLY EMPTY SPACE. ...
... Rutherford found that the only way to explain the deflections was to picture an atom with a tiny nucleus in which positive charge existed and nearly all the mass existed; And the electrons were some distance away from the nucleus. In other words, AN ATOM IS MOSTLY EMPTY SPACE. ...
Chapter 3 Nuclear Radiation
... In positron emission, • a proton is converted to a neutron and a positron. ...
... In positron emission, • a proton is converted to a neutron and a positron. ...
john dalton!! - Hawk Chemistry
... • He was born September 6th, 1766 in Eaglesfield in Cumberland. • He died on July 27th, ...
... • He was born September 6th, 1766 in Eaglesfield in Cumberland. • He died on July 27th, ...
Concept Review 3.1 Introduction to Nuclear
... Using Einstein’s equation E=mc2, we can actually calculate the energy released when a nucleus is formed from nucleons! This is called the Nuclear Binding Energy (It can also be thought of as the amount of energy required to break down the nucleus; therefore, the nuclear binding energy is also a meas ...
... Using Einstein’s equation E=mc2, we can actually calculate the energy released when a nucleus is formed from nucleons! This is called the Nuclear Binding Energy (It can also be thought of as the amount of energy required to break down the nucleus; therefore, the nuclear binding energy is also a meas ...
Chapter 1: The Mole
... Mass Number (A) Atomic Number (Z) Z = number of protons. A = Z + number of neutrons. ...
... Mass Number (A) Atomic Number (Z) Z = number of protons. A = Z + number of neutrons. ...
Name Objective 1: Matter and Energy C3H8 + 5O2 → 3CO2 + 4H2O
... c. The number of chlorine atoms is unequal in the reactants and products. d. The number of reactant atoms is equal to the number of product atoms. ...
... c. The number of chlorine atoms is unequal in the reactants and products. d. The number of reactant atoms is equal to the number of product atoms. ...
2nd Semester Review
... 5. According to current atomic theory, what is the best description for the location of the electrons? A. Orbiting in perfect rings around the nucleus B. A cloud around the nucleus showing where an electron is likely to be 6. Complete the following table: Element Group or Column # Period or Row # # ...
... 5. According to current atomic theory, what is the best description for the location of the electrons? A. Orbiting in perfect rings around the nucleus B. A cloud around the nucleus showing where an electron is likely to be 6. Complete the following table: Element Group or Column # Period or Row # # ...
BASIC CHEMISTRY
... Biology Depends on Chemistry In our biosphere, everything is made of atoms Through the interaction of chemicals we can better understand our biosphere Give an example from what we have already done in Bio. ...
... Biology Depends on Chemistry In our biosphere, everything is made of atoms Through the interaction of chemicals we can better understand our biosphere Give an example from what we have already done in Bio. ...
Ch 21.1 Study Guide
... (a) matter is lost and energy is taken in. (b) matter is lost and energy is released. (c) matter is gained and energy is taken in. (d) matter is gained and energy is released. 5. _____ For atoms of a given mass number, those with greater mass defects, have (a) smaller binding energies per nucleon. ( ...
... (a) matter is lost and energy is taken in. (b) matter is lost and energy is released. (c) matter is gained and energy is taken in. (d) matter is gained and energy is released. 5. _____ For atoms of a given mass number, those with greater mass defects, have (a) smaller binding energies per nucleon. ( ...
Document
... 19. Each substance to the right of the arrow in a chemical equation is a ________________. 20. An atom that has a +2 oxidation number has ______________________ 21. The __________ _____________ tells you how many electrons an atom must gain, lose, or share to become stable. 22. Numbers that precede ...
... 19. Each substance to the right of the arrow in a chemical equation is a ________________. 20. An atom that has a +2 oxidation number has ______________________ 21. The __________ _____________ tells you how many electrons an atom must gain, lose, or share to become stable. 22. Numbers that precede ...
Notes on Atomic Structure atoms
... same proportions (by mass and by number) of its elements This means a given compound always has the same composition, regardless of where it came from. ...
... same proportions (by mass and by number) of its elements This means a given compound always has the same composition, regardless of where it came from. ...
Section 19.1 Radioactivity
... A Review of Atomic Terms • nucleons – particles found in the nucleus of an atom – neutrons – protons • atomic number (Z) – number of protons in the nucleus • mass number (A) – sum of the number of protons and neutrons • isotopes – atoms with identical atomic numbers but different mass numbers • nucl ...
... A Review of Atomic Terms • nucleons – particles found in the nucleus of an atom – neutrons – protons • atomic number (Z) – number of protons in the nucleus • mass number (A) – sum of the number of protons and neutrons • isotopes – atoms with identical atomic numbers but different mass numbers • nucl ...
35Nuclear.old
... A. Produce energy, because the mass of the product is less than the mass of the input B. Produce energy, because the mass of the product is more than the mass of the input C. Require energy, because the mass of the product is less than the mass of the input D. Require energy, because the mass of the ...
... A. Produce energy, because the mass of the product is less than the mass of the input B. Produce energy, because the mass of the product is more than the mass of the input C. Require energy, because the mass of the product is less than the mass of the input D. Require energy, because the mass of the ...
CH_8_nucleus_new
... Rutherford found that the only way to explain the deflections was to picture an atom with a tiny nucleus in which positive charge existed and nearly all the mass existed; And the electrons were some distance away from the nucleus. In other words, AN ATOM IS MOSTLY EMPTY SPACE. ...
... Rutherford found that the only way to explain the deflections was to picture an atom with a tiny nucleus in which positive charge existed and nearly all the mass existed; And the electrons were some distance away from the nucleus. In other words, AN ATOM IS MOSTLY EMPTY SPACE. ...
Radioactivity2015
... particles. They can only be stopped by several centimeters of lead or more than a meter of concrete. In fact, gamma rays can pass right through the human body. Gamma rays often accompany other processes of decay such as alpha or beta. process. 92U ...
... particles. They can only be stopped by several centimeters of lead or more than a meter of concrete. In fact, gamma rays can pass right through the human body. Gamma rays often accompany other processes of decay such as alpha or beta. process. 92U ...
The Chemical Basis of Life
... Isotopes of an element – Different forms of an element with the same atomic number but with different mass numbers – The atoms of some isotopes are stable – Other isotopes are radioactive, having unstable atoms that spontaneously break apart (decay) to form other atoms – When radioactive atoms decay ...
... Isotopes of an element – Different forms of an element with the same atomic number but with different mass numbers – The atoms of some isotopes are stable – Other isotopes are radioactive, having unstable atoms that spontaneously break apart (decay) to form other atoms – When radioactive atoms decay ...
Complexation Reactions In Nuclear Separations
... neptunium is that the long-lived 237Np isotope readily forms 237NpO2+ that behaves much like an alkali metal, forming few complexes and moving easily with water through the environment. Its low charge results in very little sorption on common minerals that tend to sorb many of the fission products, ...
... neptunium is that the long-lived 237Np isotope readily forms 237NpO2+ that behaves much like an alkali metal, forming few complexes and moving easily with water through the environment. Its low charge results in very little sorption on common minerals that tend to sorb many of the fission products, ...
Nickel 28 Ni 58.693
... Matter can be broken down into its simple parts called __________. Each element on the periodic table has its own ___________. How many elements can be found naturally? ...
... Matter can be broken down into its simple parts called __________. Each element on the periodic table has its own ___________. How many elements can be found naturally? ...
CHAPTER 1 Practice Exercises 1.1 12.3 g Cd 1.3 26.9814 u 1.5
... An element is a chemical species comprised of only a single type of atom. A compound is a chemical species comprised of two or more elements in a definite and unchanging proportion. A reactant is a chemical species which is transformed in a chemical reaction A chemical reaction is a process whereby ...
... An element is a chemical species comprised of only a single type of atom. A compound is a chemical species comprised of two or more elements in a definite and unchanging proportion. A reactant is a chemical species which is transformed in a chemical reaction A chemical reaction is a process whereby ...
Ch. 8 Notes (Chemical Reactions) Teacher 2010
... • There are two types of Nuclear reactions, ________________ – Fission reactions involve a heavy nucleus that will split into two or three pieces. – Fusion reactions involve two light nuclei that combine into a ...
... • There are two types of Nuclear reactions, ________________ – Fission reactions involve a heavy nucleus that will split into two or three pieces. – Fusion reactions involve two light nuclei that combine into a ...
Combustion and Nuclear Reactions
... Fission is the splitting of a large atom into two or more smaller ones. ...
... Fission is the splitting of a large atom into two or more smaller ones. ...
希臘 - 中正大學化生系
... 3. The arrangement of the elements in groups of elements in the order of their atomic weights corresponds to their so-called valencies, as well as, to some extent, to their distinctive chemical properties; as is apparent among other series in that of Li, Be, B, C, N, O, and F. 4. The magnitude of th ...
... 3. The arrangement of the elements in groups of elements in the order of their atomic weights corresponds to their so-called valencies, as well as, to some extent, to their distinctive chemical properties; as is apparent among other series in that of Li, Be, B, C, N, O, and F. 4. The magnitude of th ...
nuclear test 2006
... Carbon Dating is a technique used to determine the age of organic material. The activity (rate of decay) of 14C atoms in the sample is measured. 14C has a half life of 5730 years. a) Explain how the activity of 14C in an organic sample can be used to determine its age. ...
... Carbon Dating is a technique used to determine the age of organic material. The activity (rate of decay) of 14C atoms in the sample is measured. 14C has a half life of 5730 years. a) Explain how the activity of 14C in an organic sample can be used to determine its age. ...