Energy Atoms and Elements Practice Problems
... B) An electron has a negative charge and a mass of approximately 1 amu. C) A proton has a positive charge and a mass of approximately 2 amu. D) A proton has a positive charge and a mass of approximately 1 amu. ...
... B) An electron has a negative charge and a mass of approximately 1 amu. C) A proton has a positive charge and a mass of approximately 2 amu. D) A proton has a positive charge and a mass of approximately 1 amu. ...
Chemistry I Final Exam Review Problems 2016
... ____ 69. A gas occupies a volume of 2.4 L at 14.1 kPa. What volume will the gas occupy at 84.6 kPa? a. 497 L c. 14 L b. 2.5 L d. 0.40 L ____ 70. At a certain temperature and pressure, 0.20 mol of carbon dioxide has a volume of 3.1 L. A 3.1-L sample of hydrogen at the same temperature and pressure _ ...
... ____ 69. A gas occupies a volume of 2.4 L at 14.1 kPa. What volume will the gas occupy at 84.6 kPa? a. 497 L c. 14 L b. 2.5 L d. 0.40 L ____ 70. At a certain temperature and pressure, 0.20 mol of carbon dioxide has a volume of 3.1 L. A 3.1-L sample of hydrogen at the same temperature and pressure _ ...
Atoms - Red Hook Central Schools
... 400 b.c. Greeks • Greeks philosophers ponder the nature of matter: what is it made of? • Democritus: basic particle of matter = “atom” which means “indivisble”. Envisions these to be “hard spheres” • Aristotle: does not believe in atoms ...
... 400 b.c. Greeks • Greeks philosophers ponder the nature of matter: what is it made of? • Democritus: basic particle of matter = “atom” which means “indivisble”. Envisions these to be “hard spheres” • Aristotle: does not believe in atoms ...
Chemistry 101 Chapter 4 Elements, Atoms, and Ions = =
... Natural states of the elements: some elements consist of single atoms and they are found in an isolated state (for example, Ar and He). They are called monatomic elements. Some elements are diatomic and they consist of two atoms. The atoms of these elements have special affinities for each other and ...
... Natural states of the elements: some elements consist of single atoms and they are found in an isolated state (for example, Ar and He). They are called monatomic elements. Some elements are diatomic and they consist of two atoms. The atoms of these elements have special affinities for each other and ...
Learning Check Key - Mayfield City Schools
... • an unstable nucleus of U-236 forms and undergoes fission (splits) • smaller nuclei are produced such as Kr-91 and Ba-142 • neutrons are released to bombard more U-235 nuclei ...
... • an unstable nucleus of U-236 forms and undergoes fission (splits) • smaller nuclei are produced such as Kr-91 and Ba-142 • neutrons are released to bombard more U-235 nuclei ...
Lecture notes chapter 4
... Natural states of the elements: some elements consist of single atoms and they are found in an isolated state (for example, Ar and He). They are called monatomic elements. Some elements are diatomic and they consist of two atoms. The atoms of these elements have special affinities for each other and ...
... Natural states of the elements: some elements consist of single atoms and they are found in an isolated state (for example, Ar and He). They are called monatomic elements. Some elements are diatomic and they consist of two atoms. The atoms of these elements have special affinities for each other and ...
Chapter 9 Nuclear Radiation
... • the mass number of the new nucleus is the same, but the atomic number decreases by 1. ...
... • the mass number of the new nucleus is the same, but the atomic number decreases by 1. ...
Test 1 - UTC.edu
... 14. Which one of the following statements about atoms and subatomic particles is correct? A) The proton and the neutron have identical masses. B) Rutherford discovered the atomic nucleus by bombarding gold foil with electrons C) The neutron's mass is equal to that of a proton plus an electron. D) An ...
... 14. Which one of the following statements about atoms and subatomic particles is correct? A) The proton and the neutron have identical masses. B) Rutherford discovered the atomic nucleus by bombarding gold foil with electrons C) The neutron's mass is equal to that of a proton plus an electron. D) An ...
Chemistry Study Guide
... Conservation of Mass- Mass cannot be created or destroyed in a chemical reaction. ...
... Conservation of Mass- Mass cannot be created or destroyed in a chemical reaction. ...
Chemistry Study Guide
... Conservation of Mass- Mass cannot be created or destroyed in a chemical reaction. ...
... Conservation of Mass- Mass cannot be created or destroyed in a chemical reaction. ...
chapter2 - AlvarezHChem
... • Bombardment of gold foil with α particles (helium atoms minus their electrons) • Expected to see the particles pass through the foil • Found that some of the alpha particles were deflected by the foil • Led to the discovery of a region of heavy mass at the center of the atom = nucleus ...
... • Bombardment of gold foil with α particles (helium atoms minus their electrons) • Expected to see the particles pass through the foil • Found that some of the alpha particles were deflected by the foil • Led to the discovery of a region of heavy mass at the center of the atom = nucleus ...
First 9 weeks Study Guide 8th Grade
... How many: 3 C6H12O6 Elements: 3 Carbon, Hydrogen, and Oxygen Atoms: 3x6+3x12+3x6 = 72 atoms ...
... How many: 3 C6H12O6 Elements: 3 Carbon, Hydrogen, and Oxygen Atoms: 3x6+3x12+3x6 = 72 atoms ...
Review-Semester Final (Part I)
... 41. Put Sr, Cr, Fr, Al, F, Cs and P in order of: a. Largest to smallest atomic radius:____________________________________________ b. Largest to smallest electronegativity:________________________________________ c. Largest to smallest ionization energy:_________________________________________ 42. ...
... 41. Put Sr, Cr, Fr, Al, F, Cs and P in order of: a. Largest to smallest atomic radius:____________________________________________ b. Largest to smallest electronegativity:________________________________________ c. Largest to smallest ionization energy:_________________________________________ 42. ...
7.3 – Nuclear Reactions, Fission and Fusion 7.3.1 – Describe and
... 7.3 – Nuclear Reactions, Fission and Fusion 7.3.1 – Describe and give an example of an artificial transmutation 7.3.3 – Define the term unified atomic mass unit Students must be familiar with the units MeV c -2 and GeV c -2 for mass 7.3.4 - Apply the Einstein mass – energy equivalence relationship 7 ...
... 7.3 – Nuclear Reactions, Fission and Fusion 7.3.1 – Describe and give an example of an artificial transmutation 7.3.3 – Define the term unified atomic mass unit Students must be familiar with the units MeV c -2 and GeV c -2 for mass 7.3.4 - Apply the Einstein mass – energy equivalence relationship 7 ...
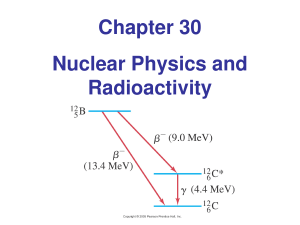
Chapter 30 Nuclear Physics and Radioactivity
... Objects older than about 60,000 years cannot be dated this way – there is too little carbon14 left. Other isotopes are useful for geologic time scale dating.Uranium238 has a halflife of 4.5x109 years, and has been used to date the oldest rocks on Earth as about 4 billion years old. ...
... Objects older than about 60,000 years cannot be dated this way – there is too little carbon14 left. Other isotopes are useful for geologic time scale dating.Uranium238 has a halflife of 4.5x109 years, and has been used to date the oldest rocks on Earth as about 4 billion years old. ...
Section G23: Atoms and Radioactivity
... (min), second (s). 7.2 describe the structure of an atom in terms of protons, neutrons and electrons and use symbols such as C 14 6 to describe particular nuclei 7.3 understand the terms atomic (proton) number, mass (nucleon) number and isotope 7.4 understand that alpha and beta particles and gamma ...
... (min), second (s). 7.2 describe the structure of an atom in terms of protons, neutrons and electrons and use symbols such as C 14 6 to describe particular nuclei 7.3 understand the terms atomic (proton) number, mass (nucleon) number and isotope 7.4 understand that alpha and beta particles and gamma ...
Atoms defy what we thought we knew! 1902 Ernest
... • Same number of _______ neutrons • Different numbers of ________ ...
... • Same number of _______ neutrons • Different numbers of ________ ...
Document
... The atomic number, Z, equals the number of protons in the nucleus. The neutron number, N, is the number of neutrons in the nucleus. The mass number, A, is the number of nucleons in the nucleus. A=Z+N “Nucleon” is a generic term used to refer to either a proton or a neutron. The mass number is not th ...
... The atomic number, Z, equals the number of protons in the nucleus. The neutron number, N, is the number of neutrons in the nucleus. The mass number, A, is the number of nucleons in the nucleus. A=Z+N “Nucleon” is a generic term used to refer to either a proton or a neutron. The mass number is not th ...
Matter Vocab Part 4
... Particle in the nucleus of an atom with a positive charge Particle in the nucleus of an atom with no charge Particle orbiting the nucleus of an atom with a negative charge Dense, central core of an atom (made of protons and neutrons) Path an electron takes around the nucleus A shell is sometimes cal ...
... Particle in the nucleus of an atom with a positive charge Particle in the nucleus of an atom with no charge Particle orbiting the nucleus of an atom with a negative charge Dense, central core of an atom (made of protons and neutrons) Path an electron takes around the nucleus A shell is sometimes cal ...
Review Chemistry KEY - cms16-17
... 4. What subatomic particle(s) are responsible for almost all of an atom’s mass? Protons and neutrons 5. Why is the overall charge of the atom neutral? The number of protons are equal to the number of electrons giving the atom a neutral charge. 6. What is the difference between the atomic number and ...
... 4. What subatomic particle(s) are responsible for almost all of an atom’s mass? Protons and neutrons 5. Why is the overall charge of the atom neutral? The number of protons are equal to the number of electrons giving the atom a neutral charge. 6. What is the difference between the atomic number and ...
atoms
... atoms have six protons, hydrogen atoms have one, and oxygen atoms have eight. The number of protons in an atom is referred to as the atomic number of that element. Atomic Symbol: The atomic symbol is one or two letters chosen to represent an element ("H" for "hydrogen," etc.). These symbols are used ...
... atoms have six protons, hydrogen atoms have one, and oxygen atoms have eight. The number of protons in an atom is referred to as the atomic number of that element. Atomic Symbol: The atomic symbol is one or two letters chosen to represent an element ("H" for "hydrogen," etc.). These symbols are used ...
Branches of Chemistry
... Inorganic chemists study the chemistry of all the elements and their compounds, except for those compounds that contain mainly carbon and hydrogen. Nuclear chemists investigate changes that happen in atomic nuclei. Organic chemists study hydrocarbons – compounds of carbon and hydrogen – and other re ...
... Inorganic chemists study the chemistry of all the elements and their compounds, except for those compounds that contain mainly carbon and hydrogen. Nuclear chemists investigate changes that happen in atomic nuclei. Organic chemists study hydrocarbons – compounds of carbon and hydrogen – and other re ...
Chapter 16 Atomic Energy
... nucleus of an atom. It differs from a chemical reaction in several ways. • One difference is that chemical reactions do not change the mass of atoms, but nuclear reactions do so by a very small amount. • A small amount of mass can change into a large amount of energy, because energy is equal to mass ...
... nucleus of an atom. It differs from a chemical reaction in several ways. • One difference is that chemical reactions do not change the mass of atoms, but nuclear reactions do so by a very small amount. • A small amount of mass can change into a large amount of energy, because energy is equal to mass ...