Need
... 1. The stability of an isotope depends on the ratio of protons to neutrons in the nucleus. Most nuclei are stable, but some are unstable. These nuclei will spontaneously decay, emitting radiation. Stable isotopes have a 1:1 ratio of protons and neutrons. Most radioactive isotopes have twice as m ...
... 1. The stability of an isotope depends on the ratio of protons to neutrons in the nucleus. Most nuclei are stable, but some are unstable. These nuclei will spontaneously decay, emitting radiation. Stable isotopes have a 1:1 ratio of protons and neutrons. Most radioactive isotopes have twice as m ...
What You Need To Know for the Chemistry Regents Exam
... 1. The stability of an isotope depends on the ratio of protons to neutrons in the nucleus. Most nuclei are stable, but some are unstable. These nuclei will spontaneously decay, emitting radiation. Stable isotopes have a 1:1 ratio of protons and neutrons. Most radioactive isotopes have twice as m ...
... 1. The stability of an isotope depends on the ratio of protons to neutrons in the nucleus. Most nuclei are stable, but some are unstable. These nuclei will spontaneously decay, emitting radiation. Stable isotopes have a 1:1 ratio of protons and neutrons. Most radioactive isotopes have twice as m ...
File
... Carried out in a Hoffman’s apparatus (shown to the right), it splits water compounds into oxygen molecules and hydrogen molecules Water Oxygen + Hydrogen H2O O2 +H2 The electrolysis reaction proves that compounds are made of more than one kind of element. Dalton’s Atomic Theory: 1. All matte ...
... Carried out in a Hoffman’s apparatus (shown to the right), it splits water compounds into oxygen molecules and hydrogen molecules Water Oxygen + Hydrogen H2O O2 +H2 The electrolysis reaction proves that compounds are made of more than one kind of element. Dalton’s Atomic Theory: 1. All matte ...
Chapter 4 Chemical Foundations: Elements, Atoms, and Ions
... • Atoms of a given element are different from those of any other element. – Carbon atoms have different chemical and physical properties than sulfur atoms. ...
... • Atoms of a given element are different from those of any other element. – Carbon atoms have different chemical and physical properties than sulfur atoms. ...
Period 10 Activity Solutions: Nuclear Reactions
... b) Make a graph on the grid below of the voltages you measured versus time elapsed. c) Use your graph to find the half-life of the capacitor discharge. _________________ 5) Radioactive decay modeled by capacitor discharge with background radiation Next, we use the equipment to produce a more realist ...
... b) Make a graph on the grid below of the voltages you measured versus time elapsed. c) Use your graph to find the half-life of the capacitor discharge. _________________ 5) Radioactive decay modeled by capacitor discharge with background radiation Next, we use the equipment to produce a more realist ...
View Transcript
... And one of the first things that you’ll notice is that the data, for the most part, fall above the line of one neutron per proton. Another way to say that is that, except for hydrogen 1, just a plain old proton, and helium-3, which is 1 proton and 2 neutrons, all are stable nuclei – have 1 or more n ...
... And one of the first things that you’ll notice is that the data, for the most part, fall above the line of one neutron per proton. Another way to say that is that, except for hydrogen 1, just a plain old proton, and helium-3, which is 1 proton and 2 neutrons, all are stable nuclei – have 1 or more n ...
Elements Elements (cont.) Elements (cont.)
... • Atoms of a given element are different from those of any other element. – C Carbon b atoms have h different diff chemical h i l andd physical h i l properties than sulfur atoms. ...
... • Atoms of a given element are different from those of any other element. – C Carbon b atoms have h different diff chemical h i l andd physical h i l properties than sulfur atoms. ...
Chemistry for Changing Times
... give carbon and hydrogen in a ratio of 3.00 g of carbon to 1.00 g of hydrogen. How much hydrogen can be made from 90.0 g CH4? ...
... give carbon and hydrogen in a ratio of 3.00 g of carbon to 1.00 g of hydrogen. How much hydrogen can be made from 90.0 g CH4? ...
final exam review packet
... 60. What two variables are related to each other in Charles’ Law: _________________ ____________________ A. When temperature is increased, volume ___________________ B. When temperature is decreased, volume ___________________ C. This law illustrates a direct / inverse relationship between temperatu ...
... 60. What two variables are related to each other in Charles’ Law: _________________ ____________________ A. When temperature is increased, volume ___________________ B. When temperature is decreased, volume ___________________ C. This law illustrates a direct / inverse relationship between temperatu ...
Chapter 4 Study Guide-Atomic Structure Define the following terms
... Chapter 4 Study Guide-Atomic Structure Define the following terms: Atom- smallest particle of an element that retains its identity in a chemical reaction Atomic Mass-weighted avg mass of the atoms in a naturally occurring sample (isotopes) Atomic Mass Unit (amu)-unit of mass of a proton or neutron ( ...
... Chapter 4 Study Guide-Atomic Structure Define the following terms: Atom- smallest particle of an element that retains its identity in a chemical reaction Atomic Mass-weighted avg mass of the atoms in a naturally occurring sample (isotopes) Atomic Mass Unit (amu)-unit of mass of a proton or neutron ( ...
History of Atomic Structure
... Very dangerous does not consist of particles Penetrates solid material including body tissues Stopped by lead or concrete ...
... Very dangerous does not consist of particles Penetrates solid material including body tissues Stopped by lead or concrete ...
Practice_Final_B
... coil is rotated by 900 so that it's plane is parallel to the field in 0.2seconds. What is the average current induced in the coil in amps? A) 0.015 B) 0.21 C) 0.90 D) 0.18 ...
... coil is rotated by 900 so that it's plane is parallel to the field in 0.2seconds. What is the average current induced in the coil in amps? A) 0.015 B) 0.21 C) 0.90 D) 0.18 ...
Made in the Stars Notes
... at room temperature except for mercury, which is a liquid. Non-metal solids are usually brittle (they break easily). Non-metals can be solids, liquids or gases at room temperature. Non-metals usually have low melting and boiling points. They are poor conductors of electricity. The exception is graph ...
... at room temperature except for mercury, which is a liquid. Non-metal solids are usually brittle (they break easily). Non-metals can be solids, liquids or gases at room temperature. Non-metals usually have low melting and boiling points. They are poor conductors of electricity. The exception is graph ...
Radiation Questions March 4th
... Once a radioactive substance is dissolved in rainwater, it can enter the food chain. Following the Chernobyl explosion, some milk supplies were found to be radioactive. If one litre of milk contaminated with iodine-131 gives a count rate of 400 counts/second, how long will it take for the count rate ...
... Once a radioactive substance is dissolved in rainwater, it can enter the food chain. Following the Chernobyl explosion, some milk supplies were found to be radioactive. If one litre of milk contaminated with iodine-131 gives a count rate of 400 counts/second, how long will it take for the count rate ...
Beta decay is a type of radioactive decay in which a beta
... charge, Z. Therefore the set of allnuclides with the same A can be introduced; these isobaric nuclides may turn into each other via beta decay. A beta-stable nucleus may undergo other kinds of radioactive decay (for example, alpha decay). In nature, most isotopes are beta-stable, but there exist a f ...
... charge, Z. Therefore the set of allnuclides with the same A can be introduced; these isobaric nuclides may turn into each other via beta decay. A beta-stable nucleus may undergo other kinds of radioactive decay (for example, alpha decay). In nature, most isotopes are beta-stable, but there exist a f ...
Chemistry Standards Review
... many large biological molecules are ionic. Large molecules (polymers), such as proteins, nucleic acids, and starch, are formed by repetitive combinations of simple subunits. The bonding characteristics of nitrogen result in the formation of a large variety of structures ranging from simple hydrocarb ...
... many large biological molecules are ionic. Large molecules (polymers), such as proteins, nucleic acids, and starch, are formed by repetitive combinations of simple subunits. The bonding characteristics of nitrogen result in the formation of a large variety of structures ranging from simple hydrocarb ...
All you need to know about Additional Science
... • Calculate the approximate radius of its nucleus (in nm), given that it will be about one ten thousandth the radius of the boron atom. Give your answer in standard form. ...
... • Calculate the approximate radius of its nucleus (in nm), given that it will be about one ten thousandth the radius of the boron atom. Give your answer in standard form. ...
The Band of Stability
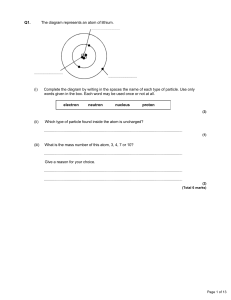
... I can explain why an unstable nuclide will undergo radioactive decay. I can describe three different types of radioactive decay in terms of balanced nuclear equations. Radioactive Decay Unstable Nuclides 1. An unstable nuclide is also known as a _____________________ nuclide. A. A __________________ ...
... I can explain why an unstable nuclide will undergo radioactive decay. I can describe three different types of radioactive decay in terms of balanced nuclear equations. Radioactive Decay Unstable Nuclides 1. An unstable nuclide is also known as a _____________________ nuclide. A. A __________________ ...
11.2 Non-renewable Energy
... break apart, the atoms nuclear energy is transformed into thermal energy. This reaction is called nuclear fission. One small pellet of uranium produces as much energy as 570 L of oil. These pellets are put into metal rods, known as fuel rods. The rods are put into a nuclear reactor where the energy ...
... break apart, the atoms nuclear energy is transformed into thermal energy. This reaction is called nuclear fission. One small pellet of uranium produces as much energy as 570 L of oil. These pellets are put into metal rods, known as fuel rods. The rods are put into a nuclear reactor where the energy ...
Document
... a) an element which has 5 electrons in each atom b) an element which has 5 electrons in its outer energy level c) an element for which the second energy level is completely filled d) an element which forms ions by gaining only one electron e) how many elements are there in the sixth period? f) the e ...
... a) an element which has 5 electrons in each atom b) an element which has 5 electrons in its outer energy level c) an element for which the second energy level is completely filled d) an element which forms ions by gaining only one electron e) how many elements are there in the sixth period? f) the e ...
Chemical Element
... The mass number of an element, A, is the number of nucleons (protons and neutrons) in the atomic nucleus. Different isotopes of a given element are distinguished by their mass numbers, which are conventionally written as a super-index on the left hand side of the atomic symbol (e.g., 238U). The rela ...
... The mass number of an element, A, is the number of nucleons (protons and neutrons) in the atomic nucleus. Different isotopes of a given element are distinguished by their mass numbers, which are conventionally written as a super-index on the left hand side of the atomic symbol (e.g., 238U). The rela ...
Atomic Structure
... • 1. All elements are composed of tiny indivisible particles called atoms. • 2. Atoms of the same element are identical. The atoms of one element are different from the atoms of another element. • 3. Atoms of different elements can physically mix together or can chemically combine in simplewhole num ...
... • 1. All elements are composed of tiny indivisible particles called atoms. • 2. Atoms of the same element are identical. The atoms of one element are different from the atoms of another element. • 3. Atoms of different elements can physically mix together or can chemically combine in simplewhole num ...
Elements PPT
... the system, how do we get the stuff we need and how do we ensure that we have enough. ...
... the system, how do we get the stuff we need and how do we ensure that we have enough. ...