The Structure of the Atom
... Protons : + charge, relative mass = 1.007 atomic mass units (amu); round to 1 Neutrons: = charge, relative mass = 1.009 atomic mass units (amu); round to 1 Electrons: - charge, relative mass = 0.0005 atomic mass units (amu); round to 0 (not factored in when figuring total mass of an atom) ...
... Protons : + charge, relative mass = 1.007 atomic mass units (amu); round to 1 Neutrons: = charge, relative mass = 1.009 atomic mass units (amu); round to 1 Electrons: - charge, relative mass = 0.0005 atomic mass units (amu); round to 0 (not factored in when figuring total mass of an atom) ...
Classification of Matter
... smaller subunits by ordinary chemical processes. 3. Elements are organized by atomic number on the periodic table. 4. Elements are identified by their symbols. ...
... smaller subunits by ordinary chemical processes. 3. Elements are organized by atomic number on the periodic table. 4. Elements are identified by their symbols. ...
Chemistry II Chapter 2 Notes
... Rutherford’s Nuclear Atom • The results of the gold foil experiment led Rutherford to realize that there was a small positive mass in the center of atoms that contained most of the mass of the atoms, called the nucleus, and electrons must orbit the nucleus. • He called the positive particles that m ...
... Rutherford’s Nuclear Atom • The results of the gold foil experiment led Rutherford to realize that there was a small positive mass in the center of atoms that contained most of the mass of the atoms, called the nucleus, and electrons must orbit the nucleus. • He called the positive particles that m ...
Atomic Theory History Presentation
... - Made up of protons, neutrons & electrons 2. Atoms can be changed from one element to another, but only by nuclear reactions 3. Atoms of the same element are not all exactly alike – they are alike in characteristics that determine chemical properties of that element but atoms of the same element ca ...
... - Made up of protons, neutrons & electrons 2. Atoms can be changed from one element to another, but only by nuclear reactions 3. Atoms of the same element are not all exactly alike – they are alike in characteristics that determine chemical properties of that element but atoms of the same element ca ...
10th Grade Chemistry X (TJ) GRADE(S)/LEVELS SUBJECT Power
... LT 1 Draw, label and describe the relative charge, mass, and location of the protons, electrons and neutrons in an atom of an element (e.g., Bohr Model, electron configuration, energy levels). LT 2 Predict the chemical properties of atoms with known number and arrangement of electrons (valence elec ...
... LT 1 Draw, label and describe the relative charge, mass, and location of the protons, electrons and neutrons in an atom of an element (e.g., Bohr Model, electron configuration, energy levels). LT 2 Predict the chemical properties of atoms with known number and arrangement of electrons (valence elec ...
05 Chemistry Basics with Flips 2011
... Pair of electrons not shared equally by 2 atoms Water = O + H oxygen has stronger “attraction” for the shared electrons than hydrogen oxygen has higher ...
... Pair of electrons not shared equally by 2 atoms Water = O + H oxygen has stronger “attraction” for the shared electrons than hydrogen oxygen has higher ...
Chapter 3
... -Matter exists as elements, compounds, mixtures, or solutions *Homogeneous matter: identical properties throughout. Ex: salt h20 ...
... -Matter exists as elements, compounds, mixtures, or solutions *Homogeneous matter: identical properties throughout. Ex: salt h20 ...
Periodic Trends & the Periodic Table
... table because putting them in their proper position would make the table very wide. • The elements in these two series are known as the inner transition elements. ...
... table because putting them in their proper position would make the table very wide. • The elements in these two series are known as the inner transition elements. ...
Chapter 2. Atoms, Molecules and Ions
... electron. However, mass of the proton is 1820 times that of the electron. Discovery of the Neutron: No charge, so harder to detect. Existence deduced from the relative masses of H and He nuclei. Refer to table on text page 43. Proton and neutrons have similar but not exactly the same mass. ...
... electron. However, mass of the proton is 1820 times that of the electron. Discovery of the Neutron: No charge, so harder to detect. Existence deduced from the relative masses of H and He nuclei. Refer to table on text page 43. Proton and neutrons have similar but not exactly the same mass. ...
Intro to Chapter 5 Development of the Periodic Table
... A. Creation of the Periodic Table by Mendeleev in 1869 is an ideal example of how scientific theory comes into being. Through random observations followed by organization of data into trends resulted in a consistent hypothesis which could explain known facts and makes correct predictions of the elem ...
... A. Creation of the Periodic Table by Mendeleev in 1869 is an ideal example of how scientific theory comes into being. Through random observations followed by organization of data into trends resulted in a consistent hypothesis which could explain known facts and makes correct predictions of the elem ...
Chapter 1: Chemistry and You
... are good representation of what to expect on the midterm, but it is not enough to just study from the review. You need to look over your notes, old review sheets, tests and quizzes, homework, etc. Ask questions!!!! You may check your answers online and I am available Tuesday, Wednesday, and Thursday ...
... are good representation of what to expect on the midterm, but it is not enough to just study from the review. You need to look over your notes, old review sheets, tests and quizzes, homework, etc. Ask questions!!!! You may check your answers online and I am available Tuesday, Wednesday, and Thursday ...
國立屏東教育大學95學年度研究所碩士班入學考試
... (A) one always corresponds to the observed structure (B) all the resonance structures are observed in various proportions (C) the observed structure is an average of the resonance forms (D) the same atoms need not be bonded to each other in all resonance forms (E) there cannot be more than two reson ...
... (A) one always corresponds to the observed structure (B) all the resonance structures are observed in various proportions (C) the observed structure is an average of the resonance forms (D) the same atoms need not be bonded to each other in all resonance forms (E) there cannot be more than two reson ...
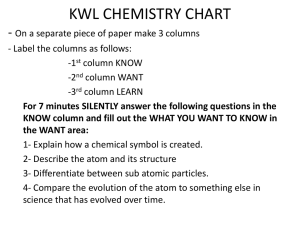
KWL chart and chem notes
... KNOW column and fill out the WHAT YOU WANT TO KNOW in the WANT area: 1- Explain how a chemical symbol is created. 2- Describe the atom and its structure 3- Differentiate between sub atomic particles. 4- Compare the evolution of the atom to something else in science that has evolved over time. ...
... KNOW column and fill out the WHAT YOU WANT TO KNOW in the WANT area: 1- Explain how a chemical symbol is created. 2- Describe the atom and its structure 3- Differentiate between sub atomic particles. 4- Compare the evolution of the atom to something else in science that has evolved over time. ...
study guide - atomic srtucture/_classification of matter
... idea that all things were made of particles too small to see. He was laughed at. In the 1800’s John Dalton proposed the idea of the “Atomic Theory”. He had 5 theories, 3 of which are still believed today. They are: 1. All matter is composed of extremely small particles too small to see 2. In reactio ...
... idea that all things were made of particles too small to see. He was laughed at. In the 1800’s John Dalton proposed the idea of the “Atomic Theory”. He had 5 theories, 3 of which are still believed today. They are: 1. All matter is composed of extremely small particles too small to see 2. In reactio ...
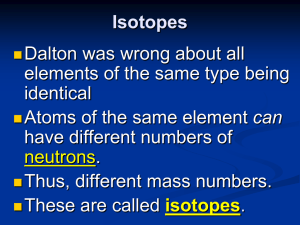
Isotopes
... Isotopes are atoms of the same element having different masses, due to varying numbers of neutrons. ...
... Isotopes are atoms of the same element having different masses, due to varying numbers of neutrons. ...
Name Class Block ______ Directions: Read the following article
... into compounds. Dalton could not look inside an atom and did not fully understand atomic structure. Despite this, his ideas inspired more than a century’s worth of research and scientific The early origins of chemistry related to alchemy. thought into the properties of atoms. Many technological adva ...
... into compounds. Dalton could not look inside an atom and did not fully understand atomic structure. Despite this, his ideas inspired more than a century’s worth of research and scientific The early origins of chemistry related to alchemy. thought into the properties of atoms. Many technological adva ...
GOB 3ed Chapter 2 part 1
... B-1 Characteristics of Groups 1A and 2A Elements that comprise a particular group have ...
... B-1 Characteristics of Groups 1A and 2A Elements that comprise a particular group have ...
Year 9 Science Revision Unit: Elements NGA PUMOTU O
... The number of electrons is always equal to the number of protons so the atom is neutral. ...
... The number of electrons is always equal to the number of protons so the atom is neutral. ...
AHSGE Review
... rows) and eighteen groups (vertical columns). Groups are together because the elements in them have similar properties and react in the same manner. Across periods (left to right), atomic radius (size) decreases, ionization energy (ease of losing an electron) increases, and electronegativity (ab ...
... rows) and eighteen groups (vertical columns). Groups are together because the elements in them have similar properties and react in the same manner. Across periods (left to right), atomic radius (size) decreases, ionization energy (ease of losing an electron) increases, and electronegativity (ab ...
Chapter 2: Atoms, Molecules, and Ions
... Fusion processes in stars have been shown to form nuclei up to 26 protons and 30 neutrons (5626Fe). ...
... Fusion processes in stars have been shown to form nuclei up to 26 protons and 30 neutrons (5626Fe). ...