Small Business Success on the Web
... Pair of electrons not shared equally by 2 atoms Water = O + H oxygen has stronger “attraction” for the shared electrons than hydrogen oxygen has higher ...
... Pair of electrons not shared equally by 2 atoms Water = O + H oxygen has stronger “attraction” for the shared electrons than hydrogen oxygen has higher ...
8th Grade: First Semester Final Review
... 1. Sample answer: The individual components of a heterogeneous mixture can be seen; the individual components of a homogeneous mixture cannot be seen. The individual components of a homogeneous mixture are evenly mixed; the individual components of a heterogeneous mixture are not evenly mixed. 2. Sa ...
... 1. Sample answer: The individual components of a heterogeneous mixture can be seen; the individual components of a homogeneous mixture cannot be seen. The individual components of a homogeneous mixture are evenly mixed; the individual components of a heterogeneous mixture are not evenly mixed. 2. Sa ...
Chapter 1 D Study Guide Answers
... 3. Electrons move around the nucleus in electron rings or shells or energy levels. 4. Atomic number is equal to the number of protons, and is unique to each element 5. The number of protons is equal to the number of electrons in a balanced atom, but not in an ION 6. The atomic mass (rounded off) is ...
... 3. Electrons move around the nucleus in electron rings or shells or energy levels. 4. Atomic number is equal to the number of protons, and is unique to each element 5. The number of protons is equal to the number of electrons in a balanced atom, but not in an ION 6. The atomic mass (rounded off) is ...
Chapter 4 Review Worksheet. Name
... atoms that have the same number of protons but different numbers of neutrons weighted average mass of the atoms in a naturally occurring sample of an element equals the number of neutrons plus the number of protons in an atom 1/12 the mass of a carbon-12 atom the number of protons in the nucleus of ...
... atoms that have the same number of protons but different numbers of neutrons weighted average mass of the atoms in a naturally occurring sample of an element equals the number of neutrons plus the number of protons in an atom 1/12 the mass of a carbon-12 atom the number of protons in the nucleus of ...
Dmitri Mendeleev
... • F2 & Cl2 gases; Br2 liquid; I2 solid • all diatomic • very reactive • react with metals to form ionic compounds • HX (X = halogen) are all acids – HF weak < HCl < HBr < HI ...
... • F2 & Cl2 gases; Br2 liquid; I2 solid • all diatomic • very reactive • react with metals to form ionic compounds • HX (X = halogen) are all acids – HF weak < HCl < HBr < HI ...
Atoms and the Periodic Table
... a. Orbitals: a region in an atom where there is a high probability of finding electrons b. There are four different kinds of orbitals Every atom has one or more valence electrons a. Valence electron: an electron in the outermost energy level of an atom ...
... a. Orbitals: a region in an atom where there is a high probability of finding electrons b. There are four different kinds of orbitals Every atom has one or more valence electrons a. Valence electron: an electron in the outermost energy level of an atom ...
NGSS Ps1. 1 Targets 1 and 2- Atoms, Elements, Molecules, and
... is made of slightly more than 100 different substances called elements. ...
... is made of slightly more than 100 different substances called elements. ...
NGSS Ps1. 1 Targets 1 and 2- Atoms, Elements, Molecules, and
... is made of slightly more than 100 different substances called elements. ...
... is made of slightly more than 100 different substances called elements. ...
Chapter 3
... by mass for example: NaCl is always 60.66% chlorine and 39.34% sodium • Law of Multiple Proportions: when two elements can form two compounds, the masses that combine are in simple whole number ratios, CO and CO2 ...
... by mass for example: NaCl is always 60.66% chlorine and 39.34% sodium • Law of Multiple Proportions: when two elements can form two compounds, the masses that combine are in simple whole number ratios, CO and CO2 ...
Unit 3 - Chemistry
... • The sum of the number of neutrons and the number of protons in a given nucleus is called the _______________. • _______________ • atoms with the same number of protons but different numbers of _______________. • Elements on the periodic table are the most common _______________ of those substance ...
... • The sum of the number of neutrons and the number of protons in a given nucleus is called the _______________. • _______________ • atoms with the same number of protons but different numbers of _______________. • Elements on the periodic table are the most common _______________ of those substance ...
- Trinity Regional School
... especially those that have small atomic radii, will bond with themselves in order to become stable and exist at a lower energy state. ...
... especially those that have small atomic radii, will bond with themselves in order to become stable and exist at a lower energy state. ...
Slide 1
... can tell you that it is arranged according to the electron configuration of the elements. – Uhm can you pass that through me one more time? • The periodic table is arranged according to the number of electrons in the outermost shell in an atom of each element. – For the average person that means wha ...
... can tell you that it is arranged according to the electron configuration of the elements. – Uhm can you pass that through me one more time? • The periodic table is arranged according to the number of electrons in the outermost shell in an atom of each element. – For the average person that means wha ...
Matter
... positive charge than negative and Cl becomes ClOpposites attract! The protons in the nucleus “miss” the “missing” electron and they stay close to it forming the ...
... positive charge than negative and Cl becomes ClOpposites attract! The protons in the nucleus “miss” the “missing” electron and they stay close to it forming the ...
Unit 4
... The resources included here provide teaching examples and/or meaningful learning experiences to address the District Curriculum. In order to address the TEKS to the proper depth and complexity, teachers are encouraged to use resources to the degree that they are congruent with the TEKS and researc ...
... The resources included here provide teaching examples and/or meaningful learning experiences to address the District Curriculum. In order to address the TEKS to the proper depth and complexity, teachers are encouraged to use resources to the degree that they are congruent with the TEKS and researc ...
Topic 1 - Periodic Table
... has a different number of neutrons. Some isotopes are radioactive; many are not. Half-life is the length of time required for half of a given sample of a radioactivy isotope to decay Electrons have little mass and a negative charge. They are located in electron clouds or probability clouds outside t ...
... has a different number of neutrons. Some isotopes are radioactive; many are not. Half-life is the length of time required for half of a given sample of a radioactivy isotope to decay Electrons have little mass and a negative charge. They are located in electron clouds or probability clouds outside t ...
Chap 03A-Atoms and Elements.pptx
... some primary elements substances have been from named which forall other substances planets, mythological are built. figures, Elements minerals, cannot colors, be broken down geographic locations into simpler and famous substances. people. Some examples are shown below: ...
... some primary elements substances have been from named which forall other substances planets, mythological are built. figures, Elements minerals, cannot colors, be broken down geographic locations into simpler and famous substances. people. Some examples are shown below: ...
Science 9 Topic 3 What Are Elements Name
... paris”, they also described the properties of many different materials. They also thought they could change lead and copper into gold. They used special symbols to prevent others from finding out their secrets. The current view of matter began with Sir Francis Bacon, who stated that all science shou ...
... paris”, they also described the properties of many different materials. They also thought they could change lead and copper into gold. They used special symbols to prevent others from finding out their secrets. The current view of matter began with Sir Francis Bacon, who stated that all science shou ...
and the atomic
... Try it Yourself! In the following pictures, there is a target hidden by a cloud. To figure out the shape of the target, we shot some beams into the cloud and recorded where the beams came out. Can you figure out the shape of the target? ...
... Try it Yourself! In the following pictures, there is a target hidden by a cloud. To figure out the shape of the target, we shot some beams into the cloud and recorded where the beams came out. Can you figure out the shape of the target? ...
+ mass isotope 2
... No charge, no mass Usually emitted in conjunction with other radiation types…why? • Lowest ionizing power, highest penetrating power requires several inches lead shielding ...
... No charge, no mass Usually emitted in conjunction with other radiation types…why? • Lowest ionizing power, highest penetrating power requires several inches lead shielding ...
Chemistry Ch 5-3 Notes: Periodic Trends
... increases as we move from left to right, because the larger number of protons holds onto electrons more strongly. The most difficult elements to remove electrons from are the noble gases (full valence) Also: The more electrons we try to remove, the harder it is to remove them, so first Ionization en ...
... increases as we move from left to right, because the larger number of protons holds onto electrons more strongly. The most difficult elements to remove electrons from are the noble gases (full valence) Also: The more electrons we try to remove, the harder it is to remove them, so first Ionization en ...
CHEMISTRY FINAL EXAM REVIEW SHEET
... Add the two half-reactions together, then cancel species which appear on both sides. ...
... Add the two half-reactions together, then cancel species which appear on both sides. ...
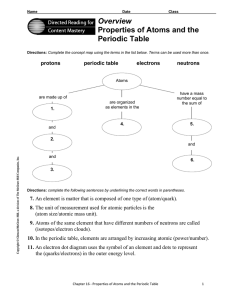
Overview Properties of Atoms and the Periodic Table
... 7. An element is matter that is composed of one type of (atom/quark). 8. The unit of measurement used for atomic particles is the (atom size/atomic mass unit). 9. Atoms of the same element that have different numbers of neutrons are called (isotopes/electron clouds). 10. In the periodic table, eleme ...
... 7. An element is matter that is composed of one type of (atom/quark). 8. The unit of measurement used for atomic particles is the (atom size/atomic mass unit). 9. Atoms of the same element that have different numbers of neutrons are called (isotopes/electron clouds). 10. In the periodic table, eleme ...
CHEMISTRY FALL FINAL PRACTICE 2016
... 11. Name an element with similar properties to magnesium. ______ How do you know? _________________ ...
... 11. Name an element with similar properties to magnesium. ______ How do you know? _________________ ...
Radioactive Isotopes and Nuclear Equations
... Atoms are composed of three subatomic particles: protons, neutrons and electrons. Protons and neutrons are found in the nucleus of an atom. The total number of protons and neutrons determines an atom’‛s mass. The number of protons defines the element. Some nuclei are unstable, so they decompose (or ...
... Atoms are composed of three subatomic particles: protons, neutrons and electrons. Protons and neutrons are found in the nucleus of an atom. The total number of protons and neutrons determines an atom’‛s mass. The number of protons defines the element. Some nuclei are unstable, so they decompose (or ...
Structure Changes of Matter
... I. All matter is composed of one or more substances (elements and/or compounds); compounds can be broken down into simpler substances: elements cannot be broken down chemically. II. All matter is composed of tiny particles, called atoms. A. Atoms of different elements are different. B. All atoms of ...
... I. All matter is composed of one or more substances (elements and/or compounds); compounds can be broken down into simpler substances: elements cannot be broken down chemically. II. All matter is composed of tiny particles, called atoms. A. Atoms of different elements are different. B. All atoms of ...