Chapter 6 Vocabulary crossword puzzle
... 3. Elements in which the highest occupied s and p sublevels are partially filled 6. Measures the ability of an atom to attract electrons when the atom is in a compound; the element named Cesium has the lowest amount, while the element named Fluorine has the highest amount 7. Term that refers to a se ...
... 3. Elements in which the highest occupied s and p sublevels are partially filled 6. Measures the ability of an atom to attract electrons when the atom is in a compound; the element named Cesium has the lowest amount, while the element named Fluorine has the highest amount 7. Term that refers to a se ...
The Chemical Basis of Life Chapter 4
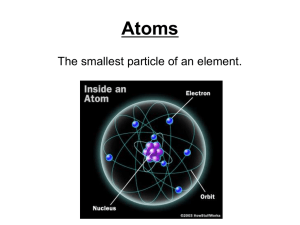
... The Atom • The atom is the smallest part of an element that retains all the characteristics of that element. • A Greek scientist named Democritus was one of the first to propose that all matter was composed of tiny particles called ...
... The Atom • The atom is the smallest part of an element that retains all the characteristics of that element. • A Greek scientist named Democritus was one of the first to propose that all matter was composed of tiny particles called ...
Elements Unit Test
... 9. A Dutch scientist by the name of Neils Bohr was the first scientist to figure out that electrons that orbit the nucleus of an atom (sometimes called shells) can only hold a certain number of electrons. Which of the following sets of numbers describe the number of electrons in the first three shel ...
... 9. A Dutch scientist by the name of Neils Bohr was the first scientist to figure out that electrons that orbit the nucleus of an atom (sometimes called shells) can only hold a certain number of electrons. Which of the following sets of numbers describe the number of electrons in the first three shel ...
C2- Topic 1: Atomic structure and the periodic table. Assessable
... - arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds - used his table to predict the existence and properties of some elements not then discovered ...
... - arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds - used his table to predict the existence and properties of some elements not then discovered ...
Notes
... -the number of protons and neutrons in an atom of an element. •The number of neutrons may vary, but the proton number remains constant. •Written as a subscript next to the element’s symbol ...
... -the number of protons and neutrons in an atom of an element. •The number of neutrons may vary, but the proton number remains constant. •Written as a subscript next to the element’s symbol ...
C2 Topic 1 Can Do Sheet
... a arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds b used his table to predict the existence and properties of some elements not then discovered 1.2 Classify elements as metals or non-metals according to their position in the pe ...
... a arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds b used his table to predict the existence and properties of some elements not then discovered 1.2 Classify elements as metals or non-metals according to their position in the pe ...
Periodic Table Vocabulary Periodic Table – a chart that organizes
... Periodic Table – a chart that organizes information about all of the known elements according to their properties. Matter – anything that has mass and volume. Element – a substance that cannot be broken down into a simpler substance by ordinary chemical means. Elemental Symbol – the single capital l ...
... Periodic Table – a chart that organizes information about all of the known elements according to their properties. Matter – anything that has mass and volume. Element – a substance that cannot be broken down into a simpler substance by ordinary chemical means. Elemental Symbol – the single capital l ...
Test 2 Review Test 2 Review (15-16)_2
... (18) ____________ How many of these elements are gases at 0 degrees Celsius? (19) ____________ How many of these elements are metalloids? (20) ____________ How many of these elements are NON-metals and solids? (21) ____________ Write the symbol of the element that would have the most similar propert ...
... (18) ____________ How many of these elements are gases at 0 degrees Celsius? (19) ____________ How many of these elements are metalloids? (20) ____________ How many of these elements are NON-metals and solids? (21) ____________ Write the symbol of the element that would have the most similar propert ...
PS 2.2
... the weighted average of the masses of the naturally occurring isotopes of an element. The atomic mass of an element can be found on the periodic table. Since it is an average, it is usually not a whole number. ...
... the weighted average of the masses of the naturally occurring isotopes of an element. The atomic mass of an element can be found on the periodic table. Since it is an average, it is usually not a whole number. ...
Chapter 4 Study Guide Physical Science 1. The word atom comes
... 2. Halogens are very reactive elements located in Group _______of the periodic table. 3. The nucleus of an atom has a(n) ____________________ electric charge. 4. Carbon is found in group ______ of the periodic table. 5. Bohr’s model of the atom compares electrons to ____________________. 6. Elements ...
... 2. Halogens are very reactive elements located in Group _______of the periodic table. 3. The nucleus of an atom has a(n) ____________________ electric charge. 4. Carbon is found in group ______ of the periodic table. 5. Bohr’s model of the atom compares electrons to ____________________. 6. Elements ...
Slide 1
... Sources for appropriate videos may include: Discovery Education and AGC Educational Media ...
... Sources for appropriate videos may include: Discovery Education and AGC Educational Media ...
Element Blocks Project
... Your assignment is to produce an element block that will have six sides, each having different information about your element. Elements will be assigned randomly. Your teacher will show you how to make the block after you have researched and obtained all the information that will go onto you block. ...
... Your assignment is to produce an element block that will have six sides, each having different information about your element. Elements will be assigned randomly. Your teacher will show you how to make the block after you have researched and obtained all the information that will go onto you block. ...
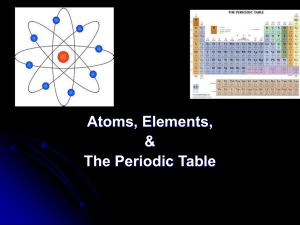
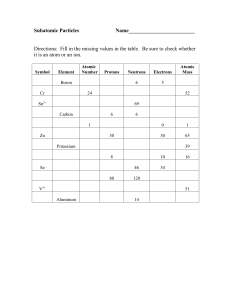
Properties of matter student notes[1]
... Protons = _______________________ charged particles in the nucleus Neutrons = _____________________ particles in the nucleus ...
... Protons = _______________________ charged particles in the nucleus Neutrons = _____________________ particles in the nucleus ...
IPC Atoms and Periodic Table
... having the same number of protons in the nucleus, or the same atomic number, but having different numbers of neutrons in the nucleus, or different atomic ...
... having the same number of protons in the nucleus, or the same atomic number, but having different numbers of neutrons in the nucleus, or different atomic ...
Intro to Atoms Clicker Questions 1. "atomos" means? 2. Atoms of one
... are located where? 6. Rutherford's proof of the proton's location in the atom came from an experiment with _______ 7. In the Bohr model of the atom, electrons are arranged how? 8. A neutron has (a) _____ charge 9. (T/F) The number of neutrons in an atom has to equal the number of protons. 10. (T/F) ...
... are located where? 6. Rutherford's proof of the proton's location in the atom came from an experiment with _______ 7. In the Bohr model of the atom, electrons are arranged how? 8. A neutron has (a) _____ charge 9. (T/F) The number of neutrons in an atom has to equal the number of protons. 10. (T/F) ...
Chapter 6 Review“The Periodic Table”
... Review“The Periodic Table” 1. How is the number of neutrons in the nucleus of an atom calculated? 2. All atoms are neutral, with the number of protons equaling the ___. 3. Isotopes of the same element have different _____. 4. Using the periodic table, determine the number of neutrons in 16O. 5. What ...
... Review“The Periodic Table” 1. How is the number of neutrons in the nucleus of an atom calculated? 2. All atoms are neutral, with the number of protons equaling the ___. 3. Isotopes of the same element have different _____. 4. Using the periodic table, determine the number of neutrons in 16O. 5. What ...
Homework Geochem Test Review
... 12. What part of an atom is negative? _________ What part is positive? _________ What part is neutral? ___________ 13. What is the atomic mass? Why don’t we count the electrons when determining the ...
... 12. What part of an atom is negative? _________ What part is positive? _________ What part is neutral? ___________ 13. What is the atomic mass? Why don’t we count the electrons when determining the ...
Chap 7: Around the Room Review
... 1. The central part of an atom is called the _____ 2. A proton has a _____ charge. 3. The atomic number tells us __________. 4. Nitrogen’s atomic number is 7. An isotope of nitrogen containing 7 neutrons would be nitrogen_____. 5. How does the size of a negative ion compare to the size of the atom t ...
... 1. The central part of an atom is called the _____ 2. A proton has a _____ charge. 3. The atomic number tells us __________. 4. Nitrogen’s atomic number is 7. An isotope of nitrogen containing 7 neutrons would be nitrogen_____. 5. How does the size of a negative ion compare to the size of the atom t ...
File
... atom: The smallest particles that make up matter. proton: a subatomic particle that has a positive charge and that is located in the nucleus of an atom. (The number of protons in the nucleus is the atomic number, which determines the identity of an element.) neutron: a subatomic particle that has no ...
... atom: The smallest particles that make up matter. proton: a subatomic particle that has a positive charge and that is located in the nucleus of an atom. (The number of protons in the nucleus is the atomic number, which determines the identity of an element.) neutron: a subatomic particle that has no ...
Quiz review
... Which element in period 3 has 6 valence electrons? Which element in period 5 has only 1 electron in its 5s sublevel? Which element in period 3 has a full octet? What family of elements has only 1 valence electron? Which family of elements has a filled valence shell? Which is larger? A Lithium atom o ...
... Which element in period 3 has 6 valence electrons? Which element in period 5 has only 1 electron in its 5s sublevel? Which element in period 3 has a full octet? What family of elements has only 1 valence electron? Which family of elements has a filled valence shell? Which is larger? A Lithium atom o ...










![Properties of matter student notes[1]](http://s1.studyres.com/store/data/009076956_1-3293fc3fecf578fd34e3f0f2700d471f-300x300.png)